Review article / Open Access
DOI:2020; 2(1): 137 - 142 . doi: 10.31488/ejrm.108
Additive Effect of Inhaled Corticosteroid (ICS) On Patients with COPD Receiving Long-Acting Muscarinic Antagonist (LAMA)/Long-Acting Β2-Agonist (LABA): A Single-Center Observational Study
Yosuke Tanaka, Mizuki Yuasa, Mitsunori Hino, Masahiro Seike, Akihiko Gemma
1. Department of Respiratory Medicine, Nippon Medical School, Chiba Hokusoh Hospital, 1715 Kamagari, Inzai, Chiba 270-1694, Japan
2. Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo, 113-8603, Japan
*Corresponding author: Y Tanaka, MD, PhD, Department of Respiratory Medicine, Chiba-Hokusoh Hospital, Nippon Medical School, 1715 Kamagari, Inzai, Chiba 270-1694, Japan.
Abstract
Background: To evaluate the efficacy of inhaled corticosteroid (ICS) as add-on to long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) in COPD patients. Methods: Of all COPD patients treated with LAMA/LABA (LAMA, Spiriva Respimat 2.5 g; LABA, Serevent Rotadisk 50) therapy for 4 weeks or longer in whom LABA therapy was replaced with ICS/LABA therapy (Advair Diskus 250/50) between April 2011 and April 2018, eligible patients receiving LAMA/LABA were evaluated for pulmonary function, SF-36, St. George’s Respiratory Questionnaire, COPD Assessment Test, modified Medical Research Council scores, and airway resistance, from 1 week before the day on which they had been switched from LAMA/LABA to LAMA/LABA/ICS until more than 4 but less than 5 weeks after switching, to gain insight into the effect and safety of ICS in COPD.Results: In 37 men (mean, 72.46 ± 7.75 years old) analyzed in the study, none of the parameters in pulmonary function tests were significant (mean difference in FEV1.0 from baseline, +0.0080, P = 0.68; FEV1.0%, +0.13, P = 0.92; and FVC, -0.26, P = 0.42), while the impedance-oscillation system showed significant changes in Fres (mean difference from baseline, -2.51, P < 0.0001) as well as in BP scores in SGRQ (mean difference from baseline, -7.03, P = 0.031). Conclusion: ICS as add-on to LAMA/LABA reduces airway elastic to inertial resistance ratios which may lead to structural airway improvements in COPD patients.
Keywords: chronic obstructive pulmonary disease, impedance-oscillation system (ios), airway resistance, inhaled corticosteroid (ics), long-acting muscarinic antagonist (lama), long-acting β2-agonist (laba).
Introduction
According to the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report) [1], an international guideline proposed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD), chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases. The GOLD’s guideline describes the chronic airflow limitation characteristic of COPD as being caused by a mixture of small airways disease (e.g., obstructive bronchiolitis) and parenchymal destruction (emphysema), with the relative contribution of each varying from person to person. Again, the GOLD’s guideline noted that chronic respiratory symptoms may precede the development of airflow limitation and may be associated with the development of acute respiratory events and that chronic respiratory symptoms also exist in individuals with normal spirometry and that a significant number of smokers without airflow limitation have structural evidence of lung disease manifested by the varying presence of emphysema, airway wall thickening and gas trapping.
Again, the GOLD’s global strategy focuses attention not on decreases in forced expiratory volume in 1 second (FEV1) but on the COPD Assessment Test (CAT) scores to evaluate quality of life (QOL) of patients with chronic obstructive pulmonary disease (COPD), modified Medical Research Council (mMRC) Dyspnea Scale scores to determine their severity of dyspnea, and the frequency of exacerbations they have had in the past year, recommending long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA) therapy combined with inhaled corticosteroid (ICS) in all patients except those with mild disease who have had 1 exacerbation in the past year [1].In this regard, Serevent Rotadisk, a LABA consisting mainly of salmeterol xinafoate, and Advair Diskus , a combination of salmeterol xinafoate (LABA) and fluticasone propionate (ICS), are both indicated for the treatment of COPD.To date, short of improving pulmonary function or mortality in COPD, ICS has been shown to improve the QOL of patients with COPD [2], while its down-titration has been associated with increases in FEV1 [3], thus making the role of ICS in COPD rather unclear [4-8].
Against this background, in this study, of all COPD patients who had been treated with LAMA/LABA therapy (LAMA, tiotropium bromide hydrate as Spiriva Respimat 2.5 g; LABA, salmeterol xinafoate as Serevent Rotadisk 50) for 4 weeks or longer and in whom LABA therapy was replaced with ICS/LABA therapy (Advair Diskus 250/50, fluticasone propionate 250 g/salmeterol xinafoate 50 g), the efficacy of 4-week ICS as add-on to LAMA/LABA therapy in COPD, as well as its safety, was investigated.
Methods
Study design. This was a single-center, observational study.
Subjects
Of all patients with COPD treated with LAMA/LABA (LAMA, tiotropium bromide hydrate as Spiriva Respimat 2.5 g; LABA, salmeterol xinafoate as Serevent Rotadisk 50) therapy for 4 weeks or longer and in whom LABA therapy was replaced with ICS/LABA therapy (Advair Diskus 250/50, fluticasone propionate 250 g/salmeterol xinafoate 50 g) between April 2011 and April 2018, those who met all the following inclusion criteria and who did not meet any of the following exclusion criteria were evaluated.
Inclusion criteria
Included in the study were those in whom LABA (Serevent Rotadisk 50) therapy was replaced with ICS/LABA (Advair Diskus 250/50, fluticasone propionate 250 g/salmeterol xinafoate 50 g) therapy after 4 weeks or more of LAMA/LABA therapy (LAMA, tiotropium bromide hydrate as Spiriva Respimat 2.5 g; LABA, salmeterol xinafoate as Serevent Rotadisk 50); those 40 years old or older diagnosed with COPD; past or current smokers with COPD; those with a FEV1/FVC ratio of less than 70%; and those in whom any disease associated with airflow obstruction other than COPD can be ruled out.
Exclusion criteria
Excluded from the study were those with a documented history of asthma, a bronchodilator response (BDR) to 400 g salbutamol shown as a FEV1 change of ≥ 200 mL or peripheral eosinophilia > 150 or 300, presence of typical asthma symptoms of atopy or history of IgE>170IU/ml and those confirmed to have received LAMA, LABA or ICS other than the study drug or their combination. The study was approved by the Medical Ethics Committee of Nippon Medical School. All participants gave their informed consent in writing.
Study design and measurements
At the time of diagnosis of COPD, all patients were assessed for BDR in terms of changes in FEV1 as well as for a documented history of asthma. The routine COPD management at our hospital involved assessing relevant parameters in all patients with COPD 1 week before or on the day during which they were being switched to or given any other drug as add-on and > 4 but < 5 weeks after change of their regimens when the study drug concentration was assumed to be at the trough (Figure 1, Figure 2).
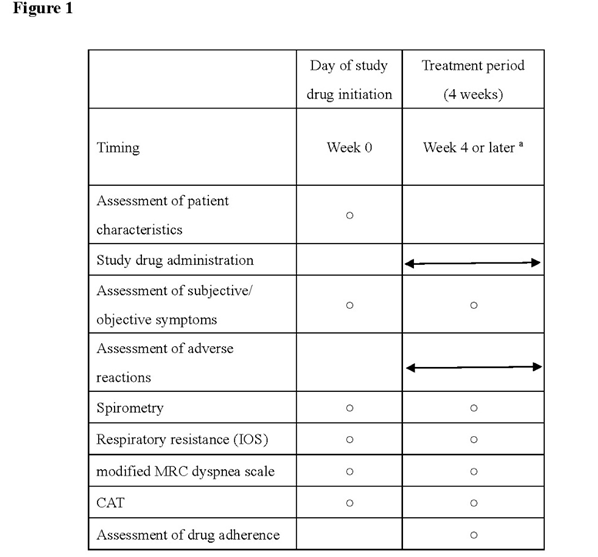
Figure 1.Routine laboratory examinations
a) All patients were instructed to visit without taking their prescribed drugs to allow them to be evaluated when the study drug concentration was assumed to be at the trough.
b) All patients who underwent the following assessments were included in the study The primary endpoints for the study were the change in airway pathology, i.e., change in FEV1 at week 4 on spirometry and respiratory resistance (both expiratory and inspiratory resistance) using a MOST graph on impulse oscillometry (IOS).
The secondary endpoints for the study included COPD Assessment Test (CAT) scores, modified Medical Research Council (mMRC) Dyspnea Scale scores, St. George’s Respiratory Questionnaire (SGRQ) scores, Short Form (36) Health Survey (SF-36) scores, and study drug safety.
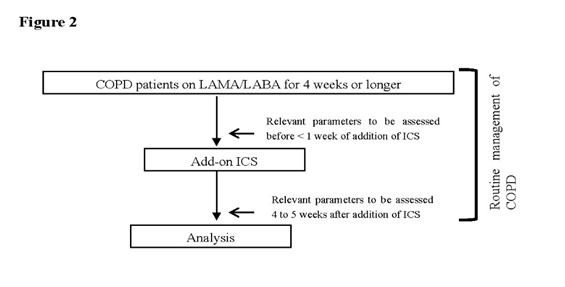
Figure 2.Routine COPD management flow at our hospital
Relevant parameters were assessed in all patients with COPD 1 week before, on the day during which they were being switched to or given any other drug as add-on, and > 4 but < 5 weeks after change of their regimens, when the study drug concentration was assumed to be at the trough. All patients were assessed for FEV1, FVC, VC as well as for IOS, CAT scores, mMRC Dyspnea Scale scores, SGRQ scores, SF-36 scores, and study drug safety.
Endpoints for the study
The primary endpoint for the study was the change in airway pathology, i.e., change in FEV1 at week 4 on spirometry and respiratory resistance (both expiratory and inspiratory resistance) using a MOST graph on impulse oscillometry (IOS). The secondary endpoints for the study included COPD Assessment Test (CAT) scores, modified Medical Research Council (mMRC) Dyspnea Scale scores, St. George’s Respiratory Questionnaire (SGRQ) scores, Short Form (36) Health Survey (SF-36) scores, and study drug safety. All eligible patients receiving LAMA/LABA were evaluated for all relevant parameters from 1 week before the day on which they had been switched from LAMA/LABA to LAMA/LABA/ICS until more than 4 but less than 5 weeks after switching (Figures 1, 2).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Changes from baseline in individual outcome measures were compared before and after treatment and analyzed for statistical significance. Analysis on paired data was performed using the Mann-Whitney U test. Changes in trend over time were analyzed using the Least Squares Method. All statistical analyses were performed using JMP version 11.2.1 (SAS Institute Inc., Cary, NC). A two-sided P value of < 0.5 was considered to indicate a statistically significant change.
Study drug
The study drugs included: Spiriva Respimat 2.5 g; Serevent Rotadisk 50 g; and Advair Diskus 250/50.
Study drug dosage and administration
All patients were instructed to inhale: Spiriva Respimat 2.5 g twice per use (equivalent to tiotropium 5 g) once daily; Serevent Rotadisk 50 g once per use twice daily; and Advair Diskus 250/50 once per use twice daily.
Subject eligibility for analysis
Patients were included if they were confirmed on their electronic medical records to have received the study drug between April 2011 and April 2018.
Results
Of all COPD patients treated with LAMA/LABA therapy for 4 weeks or longer in whom LABA therapy was replaced with ICS/LABA therapy between April 2011 and April 2018, those who met all the inclusion criteria and who met none of the exclusion criteria were evaluated.
Clinical characteristics of the patients (Table 1)
While women had not been excluded from the study, all eligible patients turned out to be male and pulmonary function tests (PFT) performed after bronchodilator administration showed that their pulmonary function were within the range consistent with the diagnostic criteria for COPD in the GOLD 2018.
All eligible patients comprised those with COPD who had received ICS at the discretion of their attending physicians. Medical records or patient interviews revealed no medication nonadherence with ICS-containing triple therapy.
Table 1. Baseline characteristics of patients
| Age (years) | 72.46 ±7.75 |
| Male, n (%) | 37(100) |
| Height, cm | 163.67 ±5.25 |
| Weight, kg | 55.9 ± 67.70 |
| FEV1 (L) | 1.22 ± 0.59 |
| FEV1/FVC (%) | 44.07 ± 15.66 |
| %FEV1/pred FEV1 | 55.83 ± 23.64 |
| %VC | 92.22 ± 20.70 |
| CAT scores | 15.38 ± 9.88 |
| mMRC Dyspnea Scale scores | 1.65 ± 1.34 |
PFT
While the addition of ICS was shown to be associated with increases in FEV1 (L), FEV1/FVC (%), and FEV1/pred-FEV1, none of these PFT values were shown to be significant, with only those for FEV1/FVC showing a weak tendency toward increase.
Ios
Following addition of ICS, IOS parameters, such as R5, R20, and R5-20, all of which reflect respiratory resistance, as well as their values measured separately in the inspiratory and expiratory phases, were shown to be associated with no significant changes, and these results were shown to be consistent with those seen for respiratory reactance at 5 Hz (X5). Again, the frequency of resonance (Fres) was shown to be associated with significantly decreases, as were the frequency of resonance in the expiratory/inspiratory phases (Ex Fres/In Fres).
Symptoms
The addition of ICS was shown to be associated with improvements in the mMRC Dyspnea Scale scores, which, however, were not significant, in contrast to those shown for the CAT scores. Again, while the addition of ICS was associated with no significant changes in the SGRQ and SF-36 scores, bodily pain (BP) as part of the SF-36 was shown to be significantly improved.
Discussion
To date, various studies have demonstrated the beneficial effects of LAMA, LABA or LAMA/LABA therapy on pulmonary function, mainly airway obstruction in COPD (10-24). This study investigated whether ICS as add-on to LAMA/LABA may confer additional benefits for patients with COPD. In this study, none of the PFT parameters demonstrated significant changes, suggesting that the addition of ICS led to no direct improvement in airflow obstruction or lung volume associated with pulmonary hyperinflation in COPD. Again, the IOS parameters, such as R5, R20, and R5-20, demonstrated no significant changes, suggesting that the addition of ICS led to no change in the location or extent of pulmonary airflow obstructive lesions involved in COPD. Furthermore, as a marker for frequency dependence of resistance, X5 demonstrated no significant change, suggesting that the addition of ICS had no significant effect on pulmonary hyperinflation. Following the addition of ICS, however, there was an increase in Fres indicating a higher elastic than inertial property, leading to the airway becoming more relaxed. Coupled with the observation that no improvement was seen in such factors as R5, R20, and R5-20, which are thought to be directly linked to respiratory resistance in COPD with the addition of ICS, this finding appears to suggest that ICS favorably affected respiratory resistance and that, short of improving the organized disease, ICS led to an increase in elastic resistance thus favorably affecting the airflow obstructive lesions thought to be in the process of remodeling leading to an increase in inertial resistance as well as a decrease in elastic resistance. In earlier studies, ICS as add-on to inhaled bronchodilators, such as LAMA or LABA, are variably reported to decrease or increase the frequency of COPD exacerbations, so that there is no consensus on the effect of ICS on COPD [2-8]. In this observational study, the addition of ICS was shown to lead to no significant changes in pulmonary function and related parameters but to significant decreases in Fres on IOS alone, suggesting that ICS may offer an additive effect by interfering with such pathological conditions as are characterized by greater airway inertial than elastic resistance (e.g., remodeling) in COPD. This appears to account for the reason that discontinuation of ICS led to no significant increase in acute exacerbations but to increases in velocity of FEV1 in patients with COPD receiving ICS in the study of Magnussen et al [3], while it remains difficult to determine from this observational study involving a small number of patients whether or not ICS as add-on to LABA/LAMA may lead to decreases in frequency of COPD exacerbations as reported earlier [4,5].
Table 2.Changes in relevant parameters before and after addition of ICS
| Pre-ICS | Post-ICS | Mean difference | P value | |
|---|---|---|---|---|
| Pulmonary function test | ||||
| FEV1 (L) | 1.22 ± 0.59 | 1.23 ± 0.61 | + 0.0080 | 0.68 |
| FEV1/FVC (%) | 44.07 ± 15.66 | 44.20 ± 15.70 | + 0.13 | 0.92 |
| %FEV1/pred FEV1 | 55.83 ± 23.64 | 56.02 ± 24.16 | + 0.19 | 0.81 |
| %VC | 92.22 ± 20.70 | 92.96 ± 19.33 | + 0.75 | 0.61 |
| Symptoms | ||||
| CAT scores | 15.38 ± 9.88 | 14.84 ± 9.63 | - 0.54 | 0.20 |
| mMRC scores | 1.65 ± 1.34 | 1.51 ± 1.26 | - 0.14 | 0.23 |
| SGRQ scores | ||||
| Symptoms | 38.63 ± 17.76 | 35.33 ± 18.31 | - 3.31 | 0.23 |
| Activity | 42.32 ± 24.34 | 41.19 ± 27.42 | - 1.12 | 0.60 |
| Impact | 21.04 ± 17.48 | 22.23 ± 18.84 | + 1.19 | 0.44 |
| Total | 31.70 ± 18.23 | 31.48 ± 19.87 | - 0.22 | 0.89 |
| SF-36 | ||||
| Physical functioning | 70.27 ± 21.50 | 67.84 ± 21.43 | - 2.43 | 0.26 |
| Role physical | 67.68 ± 26.27 | 65.94 ± 30.04 | - 1.75 | 0.66 |
| Bodily pain | 78.84 ± 25.99 | 71.81 ± 27.06 | - 7.03 | 0.031* |
| General health | 48.97 ± 13.33 | 48.78 ± 17.97 | - 0.19 | 0.94 |
| Vitality | 58.18 ± 23.71 | 52.60 ± 24.30 | + 4.42 | 0.12 |
| Social functioning | 76.28 ± 28.58 | 79.19 ± 27.20 | + 2.91 | 0.33 |
| Role emotional | 68.36 ± 31.13 | 69.38 ± 32.86 | + 0.17 | 0.96 |
| Mental health | 62.53 ± 21.82 | 66.89 ± 20.89 | + 4.36 | 0.14 |
| IOS | ||||
| R5 | 3.31 ± 1.52 | 3.20 ± 1.39 | -0.11 | 0.14 |
| Ex-R5 | 3.81 ± 1.88 | 3.72 ± 1.75 | -0.091 | 0.20 |
| In-R5 | 2.80 ± 1.23 | 2.68 ± 1.11 | -0.13 | 0.19 |
| R20 | 2.47 ± 0.89 | 2.43 ± 1.04 | -0.044 | 0.58 |
| Ex-R20 | 2.63 ± 1.00 | 2.64 ± 1.20 | + 0.0097 | 0.91 |
| In-R20 | 2.31 ± 0.83 | 2.21 ± 0.93 | -0.097 | 0.23 |
| R5-20 | 0.84 ± 0.77 | 0.77 ± 0.63 | - 0.065 | 0.46 |
| Ex R5-20 | 1.18 ± 1.02 | 1.08 ± 0.87 | - 0.10 | 0.20 |
| In R5-20 | 0.49 ± 0.58 | 0.46 ± 0.53 | - 0.030 | 0.78 |
| X5 | - 1.39 ± 0.95 | - 1.25 ± 0.90 | + 0.14 | 0.19 |
| Ex X5 | - 1.91 ± 1.49 | - 1.66 ± 1.37 | + 0.24 | 0.13 |
| In X5 | - 0.83 ± 0.56 | - 0.84 ± 0.58 | - 0.0038 | 0.96 |
| Fres | 15.78 ± 6.06 | 13.28 ± 5.88 | - 2.51 | <0.0001* |
| Ex Fres | 18.35 ± 8.10 | 15.39 ± 7.45 | - 2.96 | <0.0001* |
| In Fres | 13.22 ± 5.13 | 11.17 ± 5.30 | -2.05 | 0.0002* |
Nevertheless, our study results do not appear to rule out the possibility that the use of ICS in selected patients with COPD whose pathology is characterized by greater airway inertial than elastic resistance may effectively decrease acute exacerbations of COPD, which is not inconsistent with the observation reported in the study of Watz H et al. demonstrating that the effect of ICS may be more pronounced in COPD patients with marked airway inflammation as suggested by their eosinophil count [9].
Numerous reports are available to show the effect of LAMA, LABA or LAMA/LABA in improving pulmonary function as well as the mechanisms involved [10-26]. In contrast, very few reports are available to clarify the effect ICS may have on COPD.
While this pre-post study treating 37 patients for 4 weeks is unlikely to provide additional insights into the efficacy of triple therapy in COPD, the changes in Fres in both the inspiratory and expiratory phases seen with ICS appear to suggest that ICS may have the potential to produce structural, rather than functional, changes, in COPD as a disease primarily characterized by expiratory dysfunction.
Otherwise, a further study limitation is that while women had not been excluded from the study, perhaps reflecting the fact that men account for an overwhelming proportion of smokers in Japan, eligible patients turned out to be all male, which did not allow the effectiveness of ICS as add-on to LAMA/LABA to be evaluated in women with COPD and that this observational study involved patients treated with LAMA + LABA who had subsequently received ICS at the discretion of their attending physicians, suggesting that their pre-ICS clinical characteristics may have varied widely.
Throughout the observation period in this study, no major adverse events were seen with ICS-containing triple therapy. However, given the short duration of the study involving a very small number of patients, there remain concerns over the safety of the proposed ICS-containing regimen.
Acknowledgments
The authors thank all clinical engineers in Physiological Laboratory, Chiba-Hokusoh Hospital, Nippon Medical School, for their expertise and advice.
Conclusions
Thus, study results suggest that, of the pathologies accounting for COPD, not only obstructive respiratory dysfunction may be improved with LAMA, LABA or LAMA/LABA but airway remodeling responsible for permanent airflow obstruction may also be ameliorated with ICS.
Study results demonstrated the role of ICS as add-on in patients already receiving long-acting bronchodilators, as various guidelines suggest, and that assessment of IOS in both the inspiratory and expiratory phases is useful, in that it helped identify a subpopulation of patients with COPD who might benefit from add-on ICS therapy.
Conflict of Interest statement
The authors have no conflicts of interest to declare.
Authors' contributions:
YT: participated in the conception and design of the study, and the analysis and interpretation of data.
MY: participated in the conception and design of the study, and the analysis and interpretation of data.
MH: participated in the conception and design of the study and the interpretation of the study data.
MS: participated in the conception and design of the study.
AG: participated in the conception and design of the study, the interpretation of data, drafting of the article, and critical revisions of important intellectual content.
Trial registration:
This study was registered with UMIN-CTR Clinical Trial as UMIN 000037192 to evaluate the efficacy of inhaled corticosteroid (ICS) add on long acting muscarinic antagonist (LAMA)/long acting beta2 agonist (LABA) in COPD patients
Abbreviations
COPD: Chronic obstructive Pulmonary Disease; ICS: inhaled corticosteroid; LAMA: long-acting muscarinic antagonist; LABA: long-acting β2-agonist; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; VC: vital capacity;
X5: reactance at 5 Hz; Ex X5: reactance at 5 Hz in the expiratory phase; In X5: reactance at 5 Hz in the inspiratory phase; Fres: frequency of resonance; Ex Fres: frequency of resonance in the expiratory phase
Significance of this studyWhat is already known about this subject?
While, to date, the efficacy of LAMA, LABA or LAMA/LABA in improving pulmonary function in COPD, as well as the mechanisms of action of each leading to such beneficial effects, has been demonstrated, there is no consensus on the effect of inhaled corticosteroid (ICS) on COPD with conflicting results shown in the TORCH, WISDOM, IMPACT and FLAME studies.what is the questionWhether and how inhaled corticosteroid (ICS) can provide additive effect on patients with COPD receiving long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA)What are the new findings?
Add-on ICS was evaluated for its effects on impulse oscillometry (IOS) .
IOS demonstrated changes in Fres before and after initiation of add-on ICS.
How might these results change the focus of research or clinical practice?
The change of Fres were deemed structural, rather than functional, changes in COPD as a disease primarily characterized by expiratory dysfunction, that is, changes thought likely to counteract such conditions as airway remodeling associated with high inertial/elastic resistance ratios. Thus, the study results suggest that, of the pathologies accounting for COPD, not only obstructive respiratory dysfunction may be improved with LAMA, LABA or LAMA/LABA but airway remodeling responsible for permanent airflow obstruction may also be ameliorated with ICS, which is in agreement with earlier studies reporting the efficacy of ICS in COPD.
Ethics
The study protocol was approved by the Ethics Committee of Nippon Medical School. All patients provided their informed consent in writing prior to their participation in this study, which was performed in accordance with the ethical standards of the Declaration of Helsinki (2013).
References
1. Global Strategy for the Diagnosis. Management, and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report).
2. Calverley PM, Anderson JA, Celli B, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8):775-89.
3. Magnussen H, Disse B, Rodriguez-Roisin R, et al. WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014; 371(14):1285-94.
4. Lipson DA, Barnhart F, Brealey N, et al. IMPACT Investigators. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N Engl J Med. 2018; 378(18):1671-1680.
5. Wedzicha JA, Banerji D, Chapman KR, et al. FLAME Investigators. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016; 374(23):2222-34.
6. Singh D, Papi A, Corradi M, et.al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016; 388(10048):963-73.
7. Agusti A, Calverley PM, Celli B, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122.
8. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013; 273(6):584-94.
9. Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016; 4(5):390-8..
10. Rice KL, Kunisaki KM, Niewoehner DE, et al. Role of tiotropium in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2007; 2(2): 95–105.
11. O'Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004; 23(6):832-40.
12. Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010; 104(10):1460-72.
13. Tashkin DP, Celli B, Senn S, et al. UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15):1543-54.
14. O. Kornmann, R. Dahl, S. Centanni, et al. Once-daily indacaterol vs twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J, 37 2011; pp. 273-279
15. R. Dahl, C. Fan, K. Chung, et al. Efficacy of a new once-daily long-acting inhaled β2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax, 65 2010; pp. 473-47
16. Zhou Y, Zhong NS, Li X, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017; 377(10):923-935.
17. Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013; 42(6):1484-94.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol62.5/25 mcg in COPD. Respir Med. 2013; 107(10):1538-46.
19. Beeh KM, Westerman J, Kirsten AM, et al. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015; 32:53-9.
20. Beeh KM, Korn S, Beier J, et al. Effect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respir Med. 2014; 108(4):584-92.
21. Maltais F, Singh S, Donald AC, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trials. Ther Adv Respir Dis. 2014; 8(6):169-81.
22. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013; 1(3):199-209.
23. Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015; 109(10):1312-9.
24. Mahler DA, D'Urzo A, Bateman ED, et al. INTRUST-1 and INTRUST-2 study investigators. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax. 2012; 67(9):781-8.
25. Ikeda T, Anisuzzaman AS, Yoshiki H, et al. Regional quantification of muscarinic acetylcholine receptors and β-adrenoceptors in human airways. Br J Pharmacol. 2012; 166(6):1804-14.
26. Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010; 23(4):257-67.
Received:March 02, 2020;
Accepted: April 06, 2020;
Published: : April 08, 2020.
To cite this article : :Tanaka Y, Yuasa M, Hino M, et al..Additive Effect of Inhaled Corticosteroid (ICS) On Patients with COPD Receiving Long-Acting Muscarinic Antagonist (LAMA)/Long-Acting Β2-Agonist (LABA): A Single-Center Observational Study. European Journal of Respiratory Medicine. 2020;2:1.
©Tanaka Y,et al. 2020.
