Research Article/ Open Access
DOI:10.31488/EJRM.122
Clinical Features, Pathobiology, Efficacy, and Toxicity of Tenofovir Disoproxil Fumarate and Emtricitabine for Mild to Moderate SARS-CoV-2 Infections
E.A.G. Arruda≠1, R.J. Pires-Neto≠1, M.S. Medeiros1, J. Quirino-Filho2, M. Clementino2, R.N.D.G. Gondim2, L.M.V.C. Magalhães2, K.F. Cavalcante3, V.A.F. Viana3, L. P. Mello3, D.G.L. Lima1, A.A. Santos2, P.J.C. Magalhães2, A. Havt2, C.C. Clososki4, L.L.P. Silva4, N.P. Lopes4, E. Arruda5,6, A.A.M. Lima ≠*2,6 and study group members**
1. Hospital São José de Doenças Infecciosas (HSJ), Secretaria de Saúde do Ceará (SESA), Brazil
2. INCT-Biomedicina no Semiárido Brasileiro (INCT-Biomedicina), Núcleo de Biomedicina (NUBIMED), Faculdade de Medicina, UFC, Fortaleza, CE, Brazil
3. Secretaria de Vigilância em Saúde (SVS) e Laboratórios Central de Saúde Pública (LACEN), Brazil
4. Faculdade de Ciências Farmacêuticas de Ribeirão Preto USP, Ribeirão Preto, São Paulo, Brazil
5. Faculdade de Medicina, USP-RP, Ribeirão Preto, São Paulo, Brazil
6. Rede Vírus, Ministério da Ciência, Tecnologia, Inovações e Comunicações (MCTIC), Brasília-DF, Brazil
*Corresponding author: Aldo AM Lima - Núcleo de Biomedicina, Faculdade de Medicina, Universidade Federal do Ceará. Rua Cel. Nunes de Melo, no. 1315, Rodolfo Teófilo, Fortaleza, CE, CEP 60430-270
≠E.A.G. Arruda, R.J. Pires-Neto and A.A.M. Lima contributed equally to this work.
**The complete list of members, including e-mail addresses and Institutions of the Antiretroviral Nucleotide Analogs Study Group in COVID-C19 (ARTAN-C19) has been provided at the end of the manuscript supplements.
Abstract
This study evaluated the efficacy of tenofovir disoproxil fumarate (TDF) and TDF combined with emtricitabine (FTC) in patients with COVID-19 infections (ClinicalTrials.gov #NCT04712357). We conducted a randomized, double-blind, placebo-controlled clinical trial in patients with mild to moderate respiratory infection caused by SARS-CoV-2. Patients were randomly recruited to take 10 days of TDF (300 mg/day), TDF (300 mg/day) combined with FTC (200 mg/day) or placebo vitamin C (500 mg/day). From a total of 309 patients with clinical suspicion of SARS-CoV-2, 227 met the inclusion criteria and were randomly distributed into the following groups: (a) 75 (one did not initiate treatment) in the TDF; (b) 74 in the TDF combined with FTC; and (c) 77 in the vitamin C (placebo). Fever (≥37.8°C), ageusia or dysgeusia, anosmia or dysosmia, and two or more clinical signs and symptoms were significantly associated with SARS-CoV-2 infection. There was no significant change in clinical score based on clinical signs and symptoms between treatment groups. Patients with infection by SARS-CoV-2 had higher concentrations of G-CSF, IL-1β, IL-6 and TNF-α compared to patients without infection. Patients with fever (≥37.8°C), ageusia or dysgeusia, anosmia or dysosmia, and two or more signs and symptoms, had a better prediction for the diagnosis of COVID-19. In conclusions, patients with SARS-CoV-2 showed higher and more persistent proinflammatory cytokines and chemokines profile compared to patients not infected with SARS-CoV-2. Pharmacological intervention with TDF or TDF combined with FTC did not change the clinical signs and symptoms score assessed on the seventh day in patients with SARS-CoV-2. A graphical abstract is shown in Figure 1.
Key words: tenofovir; nucleoside/nucleotide analogues; RNA-dependent RNA polymerase inhibitors; COVID-19
Introduction
Defined clinical predictors for the new coronavirus (severe acute respiratory syndrome coronavirus-2; SARS-CoV-2) infection can help guide timely therapy and develop clinical scores for research of antiviral therapy, avoid unnecessary antibiotics, and in the proper isolation and prevention of COVID-19 transmission [1-3]. Several therapeutic agents have been evaluated for the treatment of COVID-19, but only the intravenous administration of antiviral drug remdesivir has shown efficacy in reducing the duration of the disease by 26.7% in critically ill patients [4]. No data on the efficacy and toxicity of tenofovir disoproxil fumarate (TDF) in coronavirus infections is available yet [5]. Recent studies on antiretroviral drugs, such as TDF and emtricitabine (FTC) with actions against the coronavirus have suggested promising results in the specific inhibition of SARS-CoV-2 polymerase (RNA-dependent RNA polymerase: RdRp) [6-8]. In silico studies have shown that TDF binds to the RdRp of SARS-CoV-2 and inhibits in vitro infection in cell cultures [6,7]. Furthermore, the TDF/FTC combination reduced the viral load in nasopharyngeal samples from animals (ferrets), which were experimentally infected with SARS-CoV-2 [8]. These results led us to evaluate the efficacy of TDF alone or TDF combined with FTC in reducing the clinical duration of COVID-19 via a randomized, double-blind, placebo-controlled clinical trial. In addition, we evaluated cytokines, chemokines, and cell growth factors, which are associated with mild to moderate SARS-CoV-2 infection.
Material and Methods
Study design, site, and population
This was a prospective, randomized, double-blind, placebo-controlled clinical trial. The study protocol and consent form were approved by Brazilian National Research Ethics Commission (no. 34 18262.00.0000.5045) and the ClinicalTrials.gov (NCT04712357). The study was carried out in the city of Fortaleza-CE, Brazil, with 2,686,607 inhabitants. After signing the consent form, patients with mild to moderate respiratory infection and clinical suspicion of COVID-19 were invited to participate in the study.
Selection and recruitment of participants
We selected adult patients of both sexes, who met the following inclusion criteria: (a) aged between 18 and 60 years; and (b) clinical suspicion of mild to moderate COVID-19 respiratory infection. Mild to moderate respiratory infection was defined as the absence of dyspnea, respiratory distress and an O2 saturation < 95%. The exclusion criteria were: (a) the patient was already receiving study drug; (b) plan for hospitalization within the next 24 hours; (c) contraindication for the use of study drugs; (d) diagnosis of HIV or hepatitis B virus infections; (e) pregnant woman; and (f) patients with fixed residence outside the study municipality. Patients were recruited from November 9, 2020, to July 5, 2021. A total of 309 patients were enrolled for the study, and eighty-two of them were excluded based on the inclusion and exclusion criteria. Therefore, 227 patients were randomly distributed into the three treatment groups, using a random list in blocks of three sequentially permuted numbers. However, one patient did not start treatment. Thus, 75 patients received TDF (300 mg/day), 74 patients took TDF (300 mg/day) combined with FTC (200 mg/day) and 77 vitamin C (500 mg/day) for 10 days.
Study duration and intervention regimen
The patients were followed up by the medical team and other health professionals during four visits: day 1, day 7 (±3 days), day 14 (±3 days) and day 28 (±3 days), at the outpatient clinic. On the day of recruitment, demographic and clinical data, and nasopharyngeal swab, and blood samples were collected and information on adverse events (AE) and serious adverse events (SAE) was reported; in addition, study intervention drugs and treatment were initiated. On day 7, the second visit, clinical information, nasopharyngeal swab and blood sample, data on AE and SAE were collected. The same data were collected on subsequent visits.
Molecular diagnosis of SARS-CoV-2
Molecular diagnosis was performed using real-time polymerase chain reaction (RT-qPCR) test based on United States Center for Disease Control and Prevention guidelines [9]. Nasopharyngeal swabs were used for nucleic acid (NA) inactivation and isolation. RT-qPCR uniplex assays were performed with commercially obtained primers and probes for SARS-CoV-2 genes N and Orf1ab (IDT, Newark, NJ - USA).
Biomarkers in the immune-inflammatory response
IgM and IgG tests were performed using the LIAISON® SARS-CoV-2 S1/S2 IgG and IgM kit (DiaSorin, Saluggia, Italy) a chemiluminescence-based immunoassay for the quantitative determination of antibodies for SARS-CoV-2 anti-S1 and anti-S2 IgG and qualitative IgM antibodies to SARS-CoV-2 in human serum or plasma samples according to the manufacturer's protocol. For the evaluation of biomarkers of the inflammatory response, we used XMAP Luminex technology (Merck, Kenilworth, NJ, USA), including the simultaneous analysis in the same serum sample (volume of 25 µL), and a panel of protein markers of pro and anti-inflammatory and cell growth factors (IL-6, MCP-3, IL-1β, IL1-RA, IL-10, G-CSF, TNF-α, MCP-1, IFN-, IP-10), following the manufacturer’s protocol, with results expressed in pg/mL.
Adverse events and serious adverse events system
Patients involved in the study were monitored for any AE and SAE. The SAEs were registered in a specific form, and they were reported within 14 days to the Data Safety and Managing Board (DSMB). All recorded AEs and SAEs recorded were reviewed and reported according to good clinical practice procedures [10]. AEs and SAEs occurring during the study were reported in accordance with the reports and guidelines required by the US Division of Microbiology and Infectious Diseases [11] as well as to the Review Board of the Federal University of Ceará.
Sample size and statistical analysis
The estimated sample size was calculated as 73 patients for each intervention group (total = 219 patients) to achieve a statistical power of 80% (1 – Beta; type-II error) and statistical significance of p = 0.05 (alpha: type-I error) to reduce the clinical duration of the disease in the groups receiving the drugs by 20% compared to that in the group receiving vitamin C (placebo control group), with 15% losses by exit from the study (https://clincalc.com/Stats/SampleSize.aspx). Data were entered into spreadsheets and verified by two independent researchers to ensure accuracy. All data were de-identified and statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 20.0; IBMCorporation, https://www.ibm.com). We used the Mann-Whitney U test and Kruskal-Wallis test for independent samples or Wilcoxon signed rank test for paired samples and for parametric variables. We used the χ2 test or Fisher's exact test for assessing the qualitative variables. We used unadjusted and adjusted multivariate logistic regression to assess the clinical signs and symptoms associated with SARS-CoV-2 infection and determine clinical scores. All statistical tests were 2-sided with a significance level of p < 0.05.
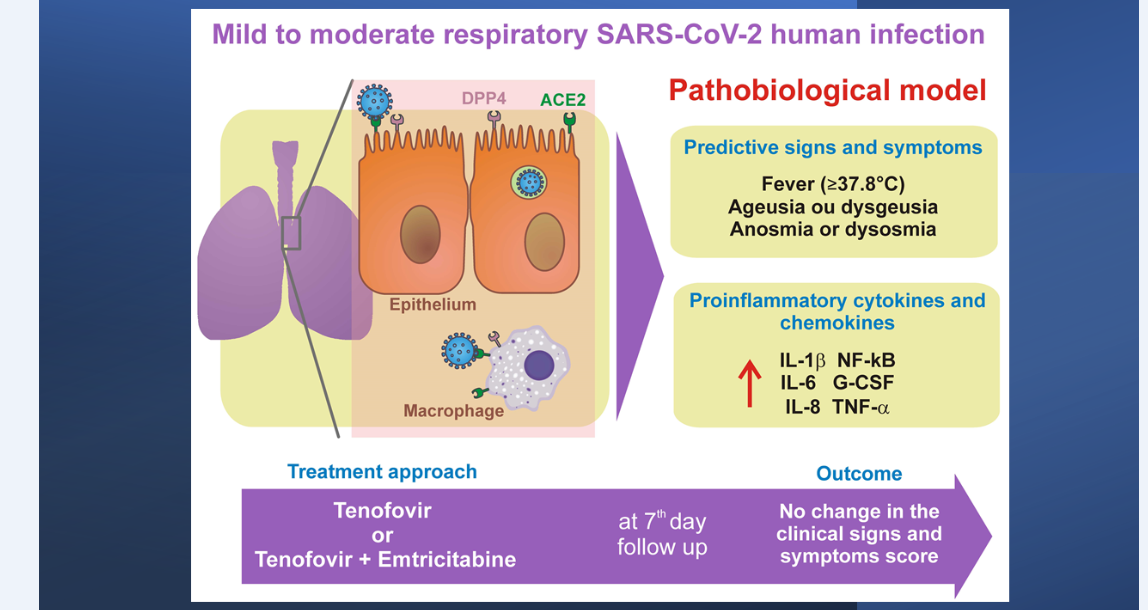
Figure 1:Graphical abstract: Patients with mild to moderate infection by SARS-CoV-2 had higher concentrations of G-CSF, IL-1β, IL-6 and TNF-α compared to patients without infection. Patients with mild to moderate respiratory infection, with fever (≥37.8°C), ageusia or dysgeusia, anosmia or dysosmia, and two or more symptoms, have a better prediction for the diagnosis of COVID-19. Pharmacological intervention with TDF or TDF combined with FTC did not change the clinical signs and symptoms score in mild to moderate respiratory infection in patients with SARS-CoV-2 compared to the vitamin C control group (placebo).
Results
Figure 2 shows the geographic location of the city of Fortaleza, state of Ceará, on the map of Brazil and the heat map of the dispersion of confirmed cases (25,683 georeferenced cases) at the peak of the second wave of COVID-19 in the city, in March 2021. The demographic and comorbidity data of the patients in the study are summarized in Table 1.
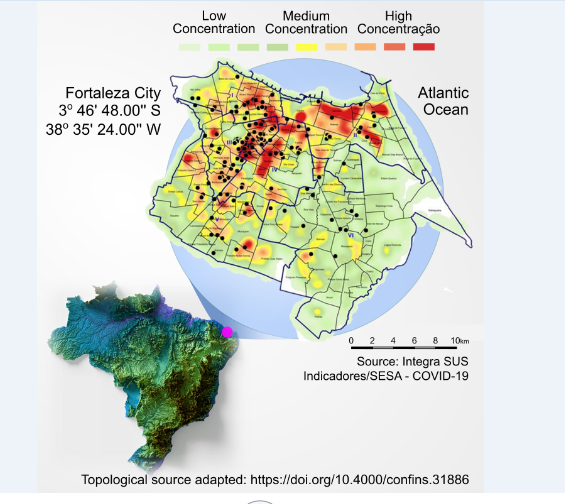
Figure 2:Map of Fortaleza city, state of Ceará, Brazil and study location. This figure shows the geographic location of the city of Fortaleza on the map of Brazil and the heat map of the dispersion of confirmed cases (25,683 georeferenced cases) at the peak of the second wave of COVID-19 in the city, in March 2021. This period coincides with the period of greatest recruitment in the clinical trial, under the predominance of the gamma variant of SARS-CoV-2. There was a large continuous agglomeration that occupied several neighborhoods such as Benfica, Damas, Bom Futuro, José Bonifácio and Parquelândia, close to Hospital São José, location of the source of recruitment of patients in the study. The map shows the spread of cases to the south of the metropolitan region of Fortaleza, CE, Brazil. The dots marked in black on the map represent the origin of patients recruited in the clinical trial, from November 9, 2020, to July 5, 2021.
Table 1:Demographic and clinical characteristics of the patients at baseline
| Characteristic | All | Tenofovir | Tenofovir plus Emtricitabine |
Placebo Vitamin C |
|---|---|---|---|---|
| (N = 226) | (N = 75) | (N = 74) | (N = 77) | |
| Age – year* | 38 ± 14.9 | 37 ± 13.8 | 36 ± 15.8 | 41 ± 14.8 |
| Male sex – no. (%) | 80 (35) | 24 (32) | 25 (34) | 31 (40) |
| Score on schooling – no. / total (%) | ||||
| 1. First grade complete | 55 / 211 (26) | 19 / 70 (27) | 20 / 70 (29) | 16 / 71 (23) |
| 2. Second grade complete | 103 / 211 (49) | 37 / 70 (53) | 34 / 70 (49) | 32 / 71 (45) |
| 3. University grade complete | 45 / 211 (21) | 12 / 70 (17) | 13 / 70 (19) | 20 / 71 (28) |
| 4. Postgraduation complete | 8 / 211 (4) | 2 / 70 (3) | 3 / 70 (4) | 3 / 71 (4) |
| Median time (IQR)** from symptom / signal onset to randomization - days |
4 (3-5) | 4 (3-5) | 4 (3-5) | 4 (3-5) |
| Total no. of symptoms and signals – median (IQR) |
6 (4-7) | 6 (4-7) | 6 (4-7) | 6 (4-7) |
| No. of coexisting conditions – no. / total (%) |
||||
| None | 152 / 224 (68) | 48 / 75 (64) | 50 / 74 (68) | 54 / 75 (72) |
| One | 53 / 224 (24) | 20 / 75 (27) | 14 / 74 (19) | 19 / 75 (25) |
| Two or more | 19 / 224 (9) | 10 / 75 (14) | 10 / 74 (14) | 2 / 75 (3) |
| Coexisting conditions – no. / total (%) |
||||
| Hypertension | 38 / 223 (17) | 15 / 75 (20) | 12 / 74 (16) | 11 / 74 (15) |
| Type 2 diabetes | 20 / 205 (10) | 10 / 69 (15) | 5 / 69 (7) | 5 / 67 (8) |
| Smoking | 16 / 220 (7) | 5 / 73 (7) | 9 / 73 (12) | 2 / 74 (3) |
| Asthma | 14 / 223 (6) | 4 / 74 (5) | 6 / 74 (8) | 4 / 75 (5) |
| Heart disease | 7 / 222 (3) | 3 / 74 (4) | 3 / 74 (4) | 2 / 74 (3) |
| Cancer | 3 / 222 (1) | 1 / 74 (1) | 1 / 73 (1) | 1 / 75 (1) |
| No. of qPCR positive for SARS-CoV-2§ - no. / total (%) |
139 / 226 (62) | 47 / 75 (63) | 45 / 74 (61) | 47 / 77 (61) |
| Viral load: SARS-CoV-2 (RNA copies / mL) – median (IQR) |
276 | 81 | 1276 | 288 |
| (2.3 - 6233.8) | (2.0 – 2503.7) | (2.0 – 12236.3) | (3.7 – 4589.0) |
* Values are means ± SD. Percentage may not total 100 because of rounding. ** IQR denotes interquartile range. § qPCR positive by
CT < 37.
+ No statistical difference between all treatment groups. Statistical tests used: Kruskal-Wallis Test or Chi-Square test Likelihood Ratio.
The selection, recruitment and follow-up visits of the patients are shown in Figure 3. Three hundred and nine patients with clinical suspicion of COVID-19 were selected and provided consent to record the demographic data and verify the inclusion and exclusion criteria for recruitment to the study. Eighty-two patients were excluded from the study because they did not meet the inclusion criteria. Hence, 227 patients were randomized to receive one of three treatments: TDF (76 patients), TDF/FTC combination (74 patients), and Vitamin C placebo (77 patients). Only one patient in the TDF group did not start the medication; therefore, 75 patients in this group started the treatment.
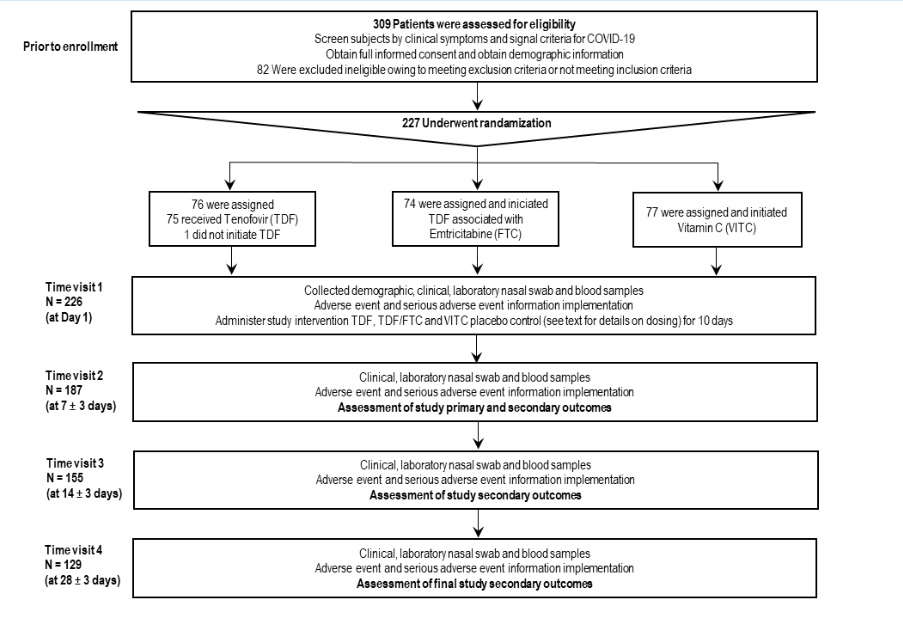
Figure3:Enrollment, randomization, and study protocol follow up. Three hundred and nine patients with clinical suspicion of COVID-19 were selected and provided consent to record the demographic data and verify the inclusion and exclusion criteria for recruitment in the study. Eightytwo patients were excluded from the study for not meeting the inclusion criteria. Two hundred twenty-seven patients were randomized to receive one of three treatments: TDF (76 patients); TDF/FTC (74 patients) and Vitamin C placebo (77 patients). Only one patient in the TDF group did not start the medication, so 75 patients in this group started the treatment
The clinical signs and symptoms in order of frequency were headache (83% [182/221]), muscle pain (73% [158/217]), rhinorrhea (71% [155/220]), cough (69% [154/222]), sore throat (68% [150/221]), weakness (66% [145/221]), fever (≥37.8°C) (53% [116/220]), ageusia or dysgeusia (31% [68/220]), anosmia or dysosmia (31% [67/220]), shortness of breath (15% [32/218]) and skin irritation (1% [1/218]) (Table 2). Thirty-two percent (72/226) had more than one sign and symptom. Patients positive for SARS-CoV-2 by RT-qPCR showed significant differences in the following signs and symptoms: sore throat (62% vs. 78%; p = 0.010; chi-square test), fever (58% vs. 43%; 0.031), ageusia or dysgeusia (36% vs. 23%; 0.045), and anosmia or dysosmia (37% vs. 19%; 0.005). As sore throat was more frequent in the negative group for SARS-CoV-2 by RT-qPCR, the clinical signs and symptoms score to assess the effectiveness of drugs in the clinical trial was based only on the three most frequent clinical signs and symptoms associated with SARS-CoV-2 (Table 2). The multivariate logistic regression model showed that anosmia or dysosmia (odds ratio = 2.70; p = 0.023) and sore throat (odds ratio = 0.45; p = 0.019) were the two most significant signs and symptoms associated with SARS-CoV-2 infection (Table 3). The model had an accuracy of 67%, a sensitivity of 82%, and a specificity of 43%. The positive and negative predictive values were 71% and 58%, respectively.
Table 2:Clinical characteristics and treatment of mild to moderate acute respiratory infections with SARS-CoV-2 infection and without SARS-CoV-2.
| Clinical signals and symptoms | All N (%) |
Patients with Laboratory RT-PCR-Confirmed SARSCoV- 2 N (%) |
Patients who Tested Negative for SARSCoV- 2 N (%) |
P values + |
|---|---|---|---|---|
| Headache | 182 / 221 (83) | 113 / 139 (81) | 69 / 82 (84) | 0.591 |
| Muscle ache | 158 / 217 (73) | 100 / 136 (74) | 58 / 81 (72) | 0.758 |
| Rhinorrhea | 155 / 220 (71) | 96 / 138 (70) | 59 / 82 (72) | 0.708 |
| Cough | 154 / 222 (69) | 101 / 139 (73) | 53 / 83 (64) | 0168 |
| Sore throat | 150 / 221 (68) | 85 / 138 (62) | 65 / 83 (78) | 0.010 |
| Weakness | 145 / 221 (66) | 86 / 138 (62) | 59 / 83 (71) | 0.184 |
| Fever (≥37.8°C) | 116 / 220 (53) | 81 / 139 (58) | 35 / 81 (43) | 0.031 |
| Ageusia or dysgeusia | 68 / 220 (31) | 49 / 137 (36) | 19 / 83 (23) | 0.045 |
| Anosmia or disosmia | 67 / 220 (31) | 51 / 137 (37) | 16 / 83 (19) | 0.005 |
| Shortness of breath | 32 / 218 (15) | 22 / 137 (16) | 10 / 81 (12) | 0.454 |
| Rash | 1 / 218 (1) | 1 / 136 (1) | 0 / 82 (0) | 0.436 |
| More than one sign or symptom | 72 / 226 (32) | 54 / 139 (39) | 18 / 83 (22) | 0.008 |
| Treatment § | N = 222 | N = 139 | N = 83 | |
| Analgesic and antipyretic | 66 (30) | 16 (12) | 50 (60) | <0.0001 |
| Antibiotic | 20 (9) | 4 (3) | 16 (19) | 0.0001 |
| Anti-inflammatory | 19 (9) | 4 (3) | 15 (18) | 0.0002 |
| Antihistamine | 16 (7) | 8 (6) | 8 (10) | 0.294 |
| Vitamins | 10 (5) | 4 (3) | 6 (7) | 0.181 |
| Ivermectin | 5 (2) | 1 (1) | 4 (5) | 0.066 |
| Antidepressant | 4 (2) | 3 (2) | 1 (1) | 1.000 |
| Nasal steroids | 4 (2) | - | 4 (5) | - |
| Syrup | 4 (2) | 1 (1) | 3 (4) | 0.148 |
+ Statistical tests used: Chi-Square test Likelihood Ratio.
§ Treatment used in three or more patients in the total.
Table 3:Multivariate logistic regression predictors of SARS-CoV-2 infections based in clinical signals and symptoms
| Clinical signals and symptoms | SARS-CoV-2 positive by RT-PCR | |||
|---|---|---|---|---|
| Odds ratio (95% CI) |
Adjusted odds ratio (95% CI) * |
P values | ||
| Fever (≥37.8°C) | 1.567 (0.877 – 2.798) | 1.422 (0.782 – 2.588) | 0.249 | |
| Sore throat | 0.434 (0.226 – 0.831) | 0.447 (0.228 – 0.875) | 0.019 | |
| Anosmia or dysosmia | 2.198 (0.970 – 4.979) | 2.699 (1.147 – 6.351) | 0.023 | |
| Ageusia or dysgeusia | 1.114 (0.506 – 2.454) | 1.033 (0.458 – 2.330) | 0.938 | |
* Adjusted by sex and age.
The main drugs used concomitantly with the drugs in the clinical trial (Table 2) were as follows: analgesics and antipyretics (30% [66/222]), antibiotics (9% [20/222]), anti-inflammatory drugs (9% [19/222]), antihistamines (7% [16/222]), vitamins (5% [10/222], and 2% for ivermectin [5/222], antidepressants [4/222], nasal steroids [4/222] and syrups [4 /222]. Patients in the SARS-CoV-2 negative group showed higher to take analgesics and antipyretics, antibiotics, and anti-inflammatory drugs (p < 0.05; chi-square test). Table 4 summarizes the variables for the two clinical scores based on the three significant predictive signs and symptoms of mild to moderate respiratory infection associated with SARS-CoV-2. When comparing the experimental groups of drugs used, there were no significant differences in the two clinical scores evaluated.
Table 4:Outcomes according to clinical scores on the ordinal scale treated population at 7th day visit with COVID-19.
| Characteristic | All | Tenofovir | Tenofovir plus Emtricitabine |
Placebo Vitamin C |
|---|---|---|---|---|
| (N = 122) | (N = 43) | (N = 38) | (N = 41) | |
| 1. Score on ordinal scale – no. (%) * | ||||
| Positive qPCR for SARS-CoV-2 | ||||
| 1. No symptom or signal | 73 (60) | 27 (63) a+ | 19 (50) a | 27 (66) a |
| 2. One symptom or signal | 13 (11) | 4 (9) a | 4 (11) a | 5 (12) a |
| 3. Two symptom or signal | 34 (28) | 11 (26) a | 15 (40) a | 8 (20) a |
| 4. Three or more symptom or signal | 2 (2) | 1 (2) a | - | 1 (2) a |
| 2. Score of symptom or signal– no. (%) | ||||
| Positive qPCR for SARS-CoV-2: | ||||
| 1. No or one symptom or signal | 86 (71) | 31 (72) a | 23 (61) a | 32 (78) a |
| 2. Two or more symptom or signal | 36 (30) | 12 (28) a | 15 (40) a | 9 (22) a |
* Percentage may not total 100 because of rounding. § qPCR positive by CT < 37.
+ Each subscript letter denotes a subset of Grupo categories whose column proportions do not differ significantly from each other at the 0.05 level
Statistical tests used: Chi-Square test Likelihood Ratio.
Patients with mild to moderate respiratory infection, positive for SARS-CoV-2 and taking the drugs in the clinical trial presented the following AEs in descending order: nausea (18% [18/122]), diarrhea (7% [9/122]), vomiting (5% [6/122]), stomach pain (3% [4/122]) and (2% [2/122]) for abdominal pain, headache, and insomnia (Table 5). The comparison between the experimental groups did not show significant differences (p >0.05). Five SAEs were recorded (four hospitalizations for COVID-19 and one hospitalization for a firearm accident), and no deaths were reported. None of these health problems were related to the study medications.
Table 5:Adverse events and serious adverse events by overall and study groups in treated patients with mild to moderate respiratory SARS-CoV-2 infection at 7th day visit.
| Parameters | All | Tenofovir | Tenofovir plus Emtricitabine |
Placebo Vitamin C |
|---|---|---|---|---|
| N = 122 (%) | N = 43 (%) | N = 38 (%) | N = 41 (%) | |
| Adverse events § | ||||
| Nausea | 18 (14.8) | 5 (11.6) a | 7 (18.4) a | 6 (14.6) a |
| Diarrhea | 9 (7.4) | 3 (7) a | 4 (10.5) a | 2 (4.9) a |
| Vomiting | 6 (4.9) | 2 (4.7) a | 2 (5.3) a | 2 (4.9) a |
| Stomachache | 4 (3.3) | 3 (7.0) | 1 (2.6) | - |
| Abdominal pain | 2 (1.6) | - | 1 (2.6) | 1 (2.4) |
| Headache | 2 (1.6) | - | 1 (2.6) | 1 (2.4) |
| Insomnia | 2 (1.6) | 1 (2.3) | 1 (2.6) | - |
| Serious adverse events | ||||
| Hospitalization | 4 (1.9) | - | 2 (2.9) | 2 (2.8) |
| Other | 1 (0.5) | 1 (1.4) | - | - |
§ Adverse events on two or more patients
+ Each subscript letter denotes a subset of Group categories whose column
proportions do not differ significantly from each other at the 0.05 level.
Statistical tests used: Chi-Square test Likelihood Ratio.
Figure 4 shows the biomarkers with significant differences between patients with mild to moderate positive and negative respiratory infections for SARS-CoV-2. Levels of G-CSF, IL-1β, IL-6 and TNF-α biomarkers were significantly higher in patients with SARS-CoV-2 infection than in patients negative for SARS-CoV-2 infection (p < 0.05; Mann-Whitney U test; see full data on supplemental Tables S1, S2, and S3). During the follow-up of biomarkers on the 14th. and 28th. days, the concentration of the biomarkers IL-10 and TNF-α, as well as IL-1β, IL-10 and MCP-3 remained higher in patients with SARS-CoV-2 infection compared to that in patients without SARS-CoV-2 infection (p < 0.05; Mann-Whitney U test). IgM concentration did not change, but IgG concentration increased significantly by the 28th day in relation to the 14th day (p < 0.05; Wilcoxon signed rank test paired samples) in patients in all treatment groups (supplement Table S4). No significant differences in immunoglobulins were observed between the experimental groups in the clinical trial.
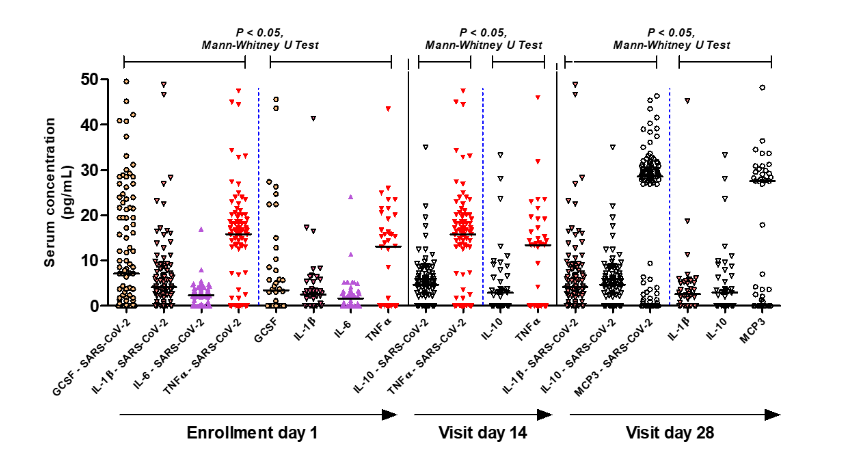
Figure 4:Cytokines, chemokines, and cell growth factor in systemic blood of patients with and without mild to moderate SARS-CoV-2 infection. This figure shows the biomarkers with significant differences between patients with mild to moderate positive and negative respiratory infection for SARS-CoV-2. Levels of G-CSF, IL-1β, IL-6 and TNF-α biomarkers are significantly higher in patients with SARS-CoV-2 compared to patients negative for SARS-CoV-2 (p < 0.05; Mann -Whitney U Test). In the follow-up of biomarkers on the 14th and 28th days, the values of the biomarkers IL-10 and TNF-α, as well as IL-1β, IL-10 and MCP-3 remained higher respectively in the times observed in patients with SARSCoV- 2 infection compared to patients without infection by SARS-CoV-2 (p < 0.05; Mann-Whitney U Test).
Discussion
Mild to moderate acute respiratory illnesses are considered the leading causes of visits to hospitals for outpatients of all ages [12]. The main signals and symptoms most associated with COVID-19 in this prospective clinical trial study were fever (≥37.8°C), ageusia or dysgeusia, and anosmia or dysosmia. Sore throat symptoms were associated with mild to moderate respiratory infections in the absence of SARS-CoV-2 infection. Multivariate logistic analysis of these symptoms adjusted for sex and age of patients showed the persistence of two symptoms, sore throat, and anosmia or dysosmia. In this model, the accuracy was 67% with sensitivity in efficacy and specificity of 82% and 43%, respectively, positive predictive value of 71% compared to 62% reliability of "practitioner's clinical intuition" and a negative predictive value of 58%. Studies before coronavirus epidemics showed consistency with this model and signs and symptoms which were more associated with influenza infection showed that fever (feeling of fever or chills), cough, myalgia and weakness were more frequent, and, in several studies, showed a predictive value for cough and fever [13,14]. A recent study from our group, performed before the pandemic, showed that severe acute respiratory infections in the absence of SARS-CoV-2 infection in the same population and geographic region studied were mainly associated with influenza A (H1N1) and influenza B viruses [15]. Thus, during the COVID-19 epidemic when SARS-CoV-2 is present in the community, a high index of suspicion of the disease is guaranteed in patients who present with an acute onset of fever (≥37.8°C), ageusia or dysgeusia, anosmia, or dysosmia. Two scores based on these symptoms (Table 4) were delineated as primary parameters and most associated with SARS-CoV-2 infection to assess the efficacy of TDF and TDF combined with FTC in patients on the seventh day of COVID-19 evolution.
This study focused on patients with suspected mild to moderate SARS-CoV-2 respiratory infection. In this sense, the pathobiology of SARS-CoV-2 infection predominates in the upper airways, the predominant site of expression in the host of angiotensin-2 converting enzyme (ACE2) receptors [16]. The S protein of SARS-CoV-2 which binds to ACE2 receptor is 10-20 times more potent than the SARS-CoV virus [17]. ACE2 is widely expressed in lung epithelial cells and other cells such as macrophages in lung tissue [18,19] and has little or no expression in human peripheral blood immune system cells [19]. Recent evidence has shown that the accessory protein encoded by ORF7a of SARS-CoV-2 is responsible for activating the transcription factor NF-kB which is associated with the expression of pro-inflammatory cytokines including IL-1alpha, IL-1beta, IL-6, IL- 8, IL-10, TNF-α and IFNβ [20]. The present work shows a profile of pro-inflammatory cytokines and chemokines such as IL-1β, IL-6, and TNF-α in addition to an increase in G-CSF in mild to moderate respiratory infections caused by SARS-CoV-2 when compared to other respiratory infections caused by seasonal respiratory viruses. A recent study comparing the cytokine and chemokines profile in patients with severe COVID-19 and severe influenza showed a similar increase in pro-inflammatory cytokines anModel of the pathobiology of mild to moderate respiratory SARS-CoV-2 human infection is shown in Figure 5. Model of the pathobiology of mild to moderate respiratory SARS-CoV-2 human infection is shown in Figure 5.
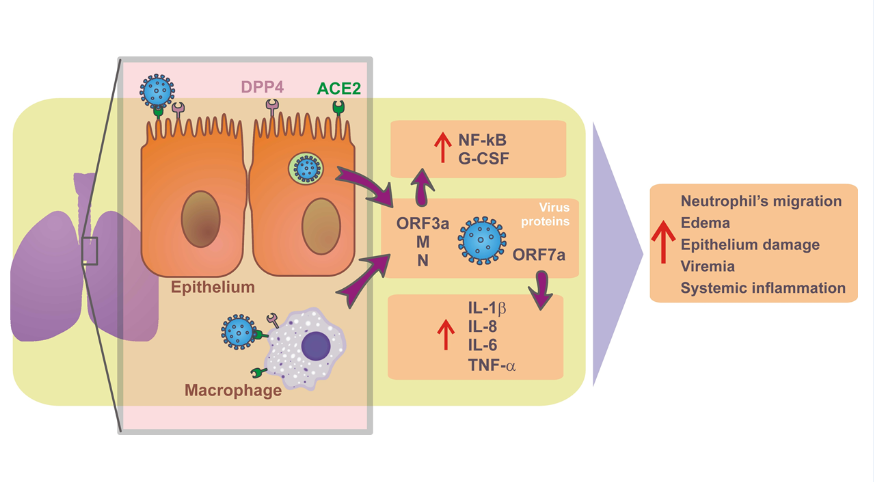
Figure 5:Model of the pathobiology of mild to moderate respiratory SARS-CoV-2 human infection. The pathobiology of SARS-CoV-2 infection predominates in the upper airways in these patients enrolled in the study protocol, the predominant site of expression in the host of angiotensin-2 converting enzyme (ACE2) receptors [17]. The S protein of SARS-CoV-2 binds to the ACE2 receptor on the pulmonary epithelial cell, allowing the virus to fuse with the host cell and supported by the action of dipeptidyl peptidase 4 (DPP4; or CD26) [17,18]. ACE2 is widely expressed in lung epithelial cells and other cells such as macrophages in lung tissue [19,20] and has little or no expression in human peripheral blood immune system cells [20]. Recent evidence has shown that the accessory protein encoded by the ORF7a of SARS-CoV-2 is responsible for activating the transcription factor NF-kB associated with the expression of pro-inflammatory cytokines including IL-1alpha, IL-1beta, IL-6, IL- 8, IL-10, TNF-alpha and IFNbeta [21]. The present work shows a profile of pro-inflammatory cytokines such as IL-1beta, IL-6 and TNF-alpha in addition to G-CSF increased in mild to moderate respiratory infections caused by SARS-CoV-2 when compared to other respiratory infections caused by seasonal respiratory viruses, suggesting a profile of cytokines produced by activation of lung tissue macrophages.
Antiviral therapies alone or in combination are important measures for the prevention and treatment of emerging viruses’ infections such as SARS-CoV-2. Although several vaccines are effective in preventing COVID-19 in various parts of the world [22-24], there is still no proven effective antiviral treatment for COVID-19. The RdRp of coronaviruses is an interesting target for antivirals [25]. Among the various drugs tested, remdesivir (adenosine analogue) was initially tested during the COVID-19 pandemic because of its high potency of inhibition of RdRp [4,26] and its wide action against several RNA viruses, including Ebola virus, hepatitis C virus, SARS-CoV and MERS-CoV [4,26]. TDF, which has a similar chemical structure to remdesivir, competitively inhibits the RdRp of HIV and hepatitis B viruses, and is well tolerated and prevents the progression of liver fibrosis [27-34], has an inhibitory effect on the RdRp of SARS-CoV-2 [7,35]. TDF is a low-cost, widely available generic drug with low toxicity and recommended in combination with FTC (cytosine analogue) or lamivudine (3TC; cytosine analogue) for effective combined antiretroviral therapy [7,28,33,34]. Several preclinical studies of these drugs for SARS-CoV-2 have been reported, including several in silico studies, which have shown the ability of TDF to bind to RdRp of SARS-CoV-2 [6, 36-44]. TDF also binds to other important viral proteins, including the papain-like protease and main or 3C-like protease (Mpro/3CLpro) [45-47], in addition to interacting with the S protein and the ACE2 receptor [48,49], suggesting multiple binding and interference targets in the replication cycle of the virus. In vitro studies have shown contradictory results regarding the efficacy of TDF; some studies have shown efficacy in reducing viral load in cell cultures and viral titers in nasopharyngeal samples in an experimental SARS-CoV-2 infection model in ferrets [6-8]. Some clinical studies suggest that TDF combined with antiretroviral therapy in patients with HIV infection has reduced the incidence of COVID-19, viral load, risk of hospitalization, and death [50-52]. These clinical data are limited and do not provide conclusive evidence suggesting the need for further clinical trials to determine the efficacy of TDF and TDF/FTC in COVID-19. In the present study, TDF and TDF/FTC combination were evaluated in patients with mild to moderate COVID-19 and revealed that there were no differences in outcomes and signs and symptoms in groups treated with TDF and TDF/FTC, nor in comparison with the group treated with the placebo. The documented AEs and SAEs were of low frequency, and there were no differences between the experimental groups.
The use of viral load and signs and symptoms scores on the seventh day of illness was a limitation of the present study. The viral load was practically zero on the seventh day of illness in all experimental groups, which makes it difficult to evaluate the antiviral action of the drugs. As the selected COVID-19 cases had mild to moderate disease, it is likely that the majority had already recovered regardless of the drugs.
Conclusions
The presence of fever (≥37.8°2,C), anosmia or dysosmia, ageusia or dysgeusia, with two or more symptoms in patients with mild to moderate respiratory infection, indicates the diagnosis of COVID-19. Pharmacological intervention with TDF and TDF/FTC did not change the clinical signs and symptoms scores in mild to moderate SARS-CoV-2 respiratory infection. Patients with mild to moderate respiratory infections positive for SARS-CoV-2 had a pro-inflammatory systemic profile with increased biomarkers G-CSF, IL-1β, IL-6 and TNF-α.
Acknowledgments
We thank the patients for their availability to participate in this study. We also thank the Ministry of Health, Brasilia, DF, Brazil for the donation of drugs used in this clinical trial.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
Conceptualization, A.A. Santos, P.J.C. Magalhães, A. Havt, N.P. Lopes, E. Arruda-Neto, A.A.M. Lima; methodology, E.A.G. Arruda, R.J. Pires-Neto, M.S. Medeiros, J. Quirino-Filho, M. Clementino, R.N.D.G. Gondim, L.M.V.C. Magalhães, K.F. Cavalcante, V.A.F. Viana, Liana Perdigão Mello, R.B Martins, A. Havt, N.P. Lopes, E. Arruda-Neto, A.A.M. Lima; formal analysis, E.A.G. Arruda, J. Quirino-Filho, M. Clementino, R.N.D.G. Gondim, A. Havt, E. Arruda-Neto, A.A.M. Lima; data curation, E.A.G. Arruda, R.J. Pires-Neto, M.S. Medeiros, J. Quirino-Filho, M. Clementino, R.N.D.G. Gondim, L.M.V.C. Magalhães, K.F. Cavalcante, V.A.F. Viana, Liana Perdigão Mello, DGL Lima, R.B Martins, A. Havt, A.A.M. Lima; writing-original draft preparation, E.A.G. Arruda, M. Clementino, R.N.D.G. Gondim, L.M.V.C. Magalhães, A.A. Santos, P.J.C. Magalhães, A. Havt, E. Arruda-Neto, A.A.M. Lima; writing-review and editing, E.A.G. Arruda, M. Clementino, R.N.D.G. Gondim, L.M.V.C. Magalhães, A.A. Santos, P.J.C. Magalhães, A. Havt, N.P. Lopes, E. Arruda-Neto, A.A.M. Lima. All authors have read and agreed to the published version of the manuscript.
ARTAN-C19 study was supported by Rede Vírus, Ministério da Ciência, Tecnologia, Inovações e Comunicações (MCTIC), Brasília-DF through CNPq, Process number: 403542/2020-0.
References
1. Pormohammad A, Ghorbani S, Khatami A, et al. Comparison of influenza type A and B with COVID‐19: A global systematic review and meta‐analysis on clinical, laboratory and radiographic findings. Rev Med Virol. 2020;e2179.
2. Maria Rosaria Barillari, Luca Bastiani, Jerome R Lechien , et al.. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: A multicenter Italian study. J Med Virol. 2021;93(2):983-994.
3. Mihaja Raberahona, Rado Rakotomalala, Etienne Rakotomijoro, et al. Clinical and epidemiological features discriminating confirmed COVID-19 patients from SARS-CoV-2 negative patients at screening centres in Madagascar. Int J Infect Dis. 2021;103:6-8.
4. JH Beigel, KM Tomashek, LE Dodd, et al. for the ACTT-1 Study Group Members.Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020;383(19):1813-1826.
5. Zanella I, Zizioli D, Castelli F, et al. Tenofovir, Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection. Pharmaceuticals (Basel). 2021;14(5):454.
6. Abdo A Elfiky. SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp) Targeting: An in silico. Perspective. J Biomol Struct Dyn. 2021;39(9):3204-3212.
7. Giuliano C Clososki, Soldi RA, Silva RM, et al. Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Braz Chem Soc 2020.
8. Su-Jin Park, Kwang-Min Yu, Young-Il Kin, Se-MI Kim et al. Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets. mBio. 2020; 11:e01114-20.
9. CDC-006-00019, Revision: 03; https://www.fda.gov/media/134922/download.
10. Good Clinical Practice Handbook. Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services; Bethesda, MD, 2019.
11. Code of Federal Regulations & ICH Guidelines, 2019.
12. Uyeki TM. Influenza. Ann Intern Med. 2017;167(5):ITC33-ITC48.
13. A S Monto, S Gravenstein, M Elliott, et al. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160(21):3243-7.
14. Long CE, Hall CB, Cunningham CK, et al. Influenza surveillance in community-dwelling elderly compared with children. Arch Fam Med. 1997;6(5):459-65.
15. Filho JQ, Junior FS, Lima TBR, et al. Perinatal Outcomes of Asynchronous Influenza Vaccination, Ceará, Brazil, 2013-2018. Emerg Infect Dis. 2021;27(9):2409-2420.
16. Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182(2):429-446.e14.
17. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263.
18. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879.
19. Xiang Song, Wei Hu, Haibo Yu, et al. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry A. 2020.
20. Su CM, Wang L, Yoo D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci Rep. 2021;11(1):13464.
21. Karaba AH, Zhou W, Hsieh LL, et al. Differential Cytokine Signatures of SARS-CoV-2 and Influenza Infection Highlight Key Differences in Pathobiology. Clin Infect Dis. 2021:ciab376.
22. Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines (accessed on 12 April 2021).
23. Golob JL, Lugogo N, Lauring AS, et al. SARS-CoV-2 vaccines: A triumph of science and collaboration. JCI Insight. 2021;6(9):e149187.
24. Kumar A, Dowling WE, Román RG, et al. Status Report on COVID-19 Vaccines Development. Curr Infect Dis Rep. 2021;23(6):9.
25. Jeong GU, Song H, Yoon GY, et al. Therapeutic Strategies against COVID-19 and Structural Characterization of SARS-CoV-2: A Review. Front. Microbiol. 2020, 11, 1723.
26. Gordon CJ, Tchesnokov EP, Woolner E, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020; 295(20):6785-6797.
27. Andrei G, Gillemot S, Topalis D, et al. The Anti–Human Immunodeficiency Virus Drug Tenofovir, a Reverse Transcriptase Inhibitor, Also Targets the Herpes Simplex Virus DNA Polymerase. J Infect Dis. 2017; 217: 790–801.
28. Buchbinder SP, Liu AY. CROI 2019: Advances in HIV prevention and plans to end the epidemic. Top. Antivir. Med. 2019; 27:8–25.
29. Celum C, Hong T, Cent A, et al. Herpes Simplex Virus Type 2 Acquisition among HIV-1–Infected Adults Treated with Tenofovir Disoproxyl Fumarate as Part of Combination Antiretroviral Therapy: Results from the ACTG A5175 PEARLS Study. J Infect Dis. 2017; 215: 907–910.
30. Chaix M.-L, Charreau I, Pintado C, et al. Effect of On-Demand Oral Pre-exposure Prophylaxis with Tenofovir/Emtricitabine on Herpes Simplex Virus-1/2 Incidence among Men Who Have Sex with Men: A Substudy of the ANRS IPERGAY Trial. Open Forum. Infect Dis. 2018, 5.
31. Marrazzo JM, Rabe L, Kelly C, et al. Tenofovir Gel for Prevention of Herpes Simplex Virus Type 2 Acquisition: Findings from the VOICE Trial. J Infect Dis. 2019; 219: 1940–1947.
32. Mu Y, Pham M, Podany AT, et al. Evaluating emtricitabine + rilpivirine + tenofovir alafenamide in combination for the treatment of HIV-infection. Expert Opin. Pharmacother. 2020; 21:389–397.
33. Santevecchi BA, Miller S, Childs-Kean LM. Doing More with Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tablet Regimen for Antiretroviral-Naïve Adults with HIV-1 Infection. Ann Pharmaco ther. 2020; 54: 1252–1259.
34. Waters L, Mehta V, Gogtay J, et al. The evidence for using tenofovir disoproxil fumarate plus lamivudine as a nucleoside analogue backbone for the treatment of HIV. J Virus Erad. 2021; 7: 100028.
35. DeJong C, Spinelli MA, Okochi H, Gandhi M. Tenofovir-based PrEP for COVID-19: an untapped opportunity? AIDS. 2021;35(9):1509-1511.
36. Copertino DC, Jr Lima BCC, Duarte RRR, et al. Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection. J Biomol Struct Dyn. 2021; 2021, 1–14.
37. Dallocchio RN, Dessì A, De Vito A, et al. Early combination treatment with existing HIV antivirals: An effective treatment for COVID-19? Eur Rev Med Pharmacol Sci. 2021; 25: 2435–2448.
38. Edited Grahl MV, Alcará, AM, de Souza ON, et al. Evaluation of drug repositioning by molecular docking of pharmaceutical resources available in the Brazilian healthcare system against SARS-CoV-2. Inform Med Unlocked 2021; 23: 100539.
39. Edited Udofia IA, Gbayo KO, Oloba-Whenu OA, et al. In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV. Netw Model Anal Health Inform Bioinform. 2021; 10: 1–12.
40. Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020; 253: 117592.
41. Hasan K, Kamruzzaman M, Bin Manjur OH, et al. Structural analogues of existing anti-viral drugs inhibit SARS-CoV-2 RNA dependent RNA polymerase: A computational hierarchical investigation. Heliyon. 2021; 7: e06435.
42. Poustforoosh A, Hashemipour H, Tüzün B, et al. Evaluation of potential anti-RNA-dependent RNA polymerase (RdRP) drugs against the newly emerged model of COVID-19 RdRP using computational methods. Biophys Chem. 2021; 272: 106564.
43. Salpini R, Alkhatib M, Costa G, et al. Key genetic elements, single and in clusters, underlying geographically dependent SARS-CoV-2 genetic adaptation and their impact on binding affinity for drugs and immune control. J Antimicrob Chemother. 2021; 76:396–412.
44. Tiwari V. Denovo designing, retro-combinatorial synthesis, and molecular dynamics analysis identify novel antiviral VTRM1.1 against RNA-dependent RNA polymerase of SARS CoV2 virus. Int J Biol Macromol. 2021;171:358–365.
45. Edited Rahman MR, Banik A, Chowdhury IM, et al. Identification of potential antivirals against SARS-CoV-2 using virtual screening method. Inform. Med Unlocked. 2021; 23:100531.
46. Feng Z, Chen M, Xue Y, et al. MCCS: A novel recognition pattern-based method for fast track discovery of anti-SARS-CoV-2 drugs. Brief Bioinform. 2021; 22: 946–962.
47. Shah B, Modi P, Sagar SR. In silico studies on therapeutic agents for COVID-19: Drug repurposing approach. Life Sci. 2020; 252: 117652.
48. Toor HG, Banerjee DI, Rath SL, et al. Computational drug re-purposing targeting the spike glycoprotein of SARS-CoV-2 as an effective strategy to neutralize COVID-19. Eur J Pharmacol. 2021; 890:173720.
49. Yun Y, Song H, Ji Y, et al. Identification of therapeutic drugs against COVID-19 through computational investigation on drug repurposing and structural modification. J Biomed Res. 2020; 34: 458–469.
50. Del Amo J, Polo R, Moreno S, et al. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy. Ann Intern Med. 2020;173:536–541.
51. Del Amo J, Polo R, Moreno S, et al. Antiretrovirals and Risk of COVID-19 Diagnosis and Hospitalization in HIV-Positive Persons. Epidemiol. 2020; 31: e49–e51.
52. Jean-Jacques Parienti, Thierry Prazuck, Laure Peyro-Saint-Paul, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. E Clin Med.2021:10099.
Received: October 11, 2021.
Accepted: November 18, 2021.
Published: November 22, 2021.
To cite this article : Arruda EAG, Pires-Neto RJ, Medeiros MS, et al Clinical, Pathobiology, Efficacy, and Toxicity of Tenofovir Disoproxil Fumarate and Emtricitabine for Mild to Moderate SARS-CoV-2 Infections. European Journal of Respiratory Medi¬cine. 2021; 3(3): 238 - 248. doi:10.31488/EJRM.122.
© 2021 Arruda EAG, et al.
