Review Article/ Open Access
DOI: 2021; 3(2): 200 - 205. doi: 10.31488/EJRM.116
Crazy-Paving Pattern: A Rare Case of Autoimmune Pulmonary Alveolar Proteinosis (PAP) with Positive Anti-GM-CSF Antibody Following Cryptococcal Infection in an otherwise Healthy Individual and Review of Literature
Wu Tzen Lim*, Singankutti Mudalige Thanuja Nilushi Priyangika, Jayasundarage Shanka Kapila Parakrama Karunarathne
1. Lyell McEwin Hospital Haydown Rd, Elizabeth Vale SA 5112, Australia
*Corresponding author: Basic Physician Trainee, Lyell McEwin Hospital Haydown Rd, Elizabeth Vale SA 5112, Australia
Abstract
Our case is a middle-aged otherwise healthy reformed smoker who developed autoimmune pulmonary alveolar proteinosis (PAP) with positive granulocyte macrophage colony-stimulating factor antibodies (anti-GM-CSF) six years following the diagnosis of cryptococcal meningitis and possible pulmonary cryptoccocoma needing whole lung lavage. Presence of anti-GM-CSF antibodies increase the risk of pulmonary and extrapulmonary opportunistic infections as well as the development of immune mediated PAP in susceptible individuals. Not all the patients with positive anti-GM-CSF antibodies and cryptococcal infection develop PAP and the exact reason for this is still unknown. On the other hand, all the patients with PAP do not require specific therapeutic intervention such as whole lung lavage. Although there were only few case reports that documented the association of cryptococcal infection with autoimmune PAP, many of these are reported pre-dated to the discovery of GM-CSF antibody. Up to now there are only three confirmed reported cases with similar clinical presentation with positive anti-GM-CSF antibodies. This could be the fourth published case with similar antecedent event prior to the diagnosis of autoimmune PAP and the second such patient who required whole lung lavage for symptom control. Diagnosis of opportunistic infection might precede the diagnosis of PAP in immunocompetent patients, and we urge all clinicians to be more vigilant especially in patients with radiological findings. Anti-GM-CSF antibodies should be measured in any healthy individuals that develop cryptococcal meningitis to expand the current database and facilitate the prediction of PAP in future.
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare disease characterised by accumulation of lipoproteinaceous material composed principally of surfactant phospholipid and apoproteins within the alveoli, resulting in impairment of gas exchange [1, 2]. It was first described by Rosen et al. in 1958 [1]. There is a wide range of clinical manifestation of PAP ranging from respiratory failure to spontaneous resolution. Autoimmune PAP is characterised by positive anti-GM-CSF antibody and presence of this antibody increase the susceptibility to opportunistic infections. We report a rare case of a middle-aged reformed smoker who was diagnosed with autoimmune PAP six years following his initial diagnosis of cryptococcal meningitis and pulmonary cryptoccocoma.
Case Report
Our patient is a 44-year-old Bhutanese cleaner, who is a reformed smoker but otherwise healthy. He was initially referred to us after an incidental finding of left apical opacity with bilateral peri-bronchial ground glass changes evident on chest computed tomography (Figure 1). This was detected when he was simultaneously diagnosed with cryptococcal meningoencephalitis in 2014 with positive C. neoformans and C. gattii on lumbar puncture. The pulmonary opacity was thought to be pulmonary dissemination from cryptococcal infection. Therefore, he was commenced on antifungal treatment and underlying immunodeficiency was excluded.
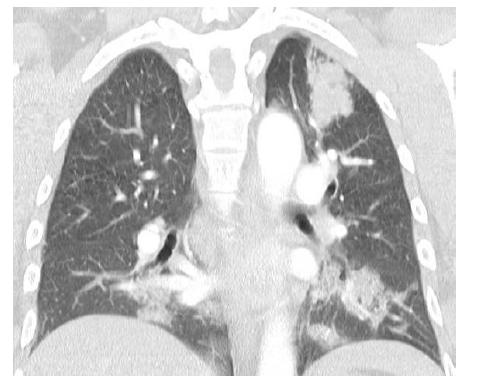
Figure1:Coronal view of CT chest showing left apical opacity suggestive of cryptoccocoma and bilateral patchy ground glass changes in 2014.commenced on antifungal treatment and underlying immunodeficiency was excluded
Upon completion of antifungal, his CT chest showed significant reduction in size of the soft tissue density in the left lung apex supporting the diagnosis of pulmonary cryptoccocoma. However, there was progression in the extensive ground-glass infiltrates throughout the lungs. Flexible bronchoscopy and biopsy ruled out malignancy, Tuberculosis and fungal infection including Pneumocystis jirovecii. No specific diagnosis made at this stage as the patient was clinically well and close observation offered. Unfortunately, since then he was lost to follow up from our care.
After six years, he was re-referred to our respiratory out -patient department with six months history of progressive worsening of dyspnoea and poor exercise tolerance, prompting further investigations. On further questioning he also reported long standing cough with whitish sputum production, loss of appetite, loss of weight and intermittent low-grade fever. No history of exposure to inorganic or organic dust and was not taking any long-term medications that can cause interstitial lung disease. On examination, he was dyspnoeic at rest, plethoric and mildly cyanosed with early finger and toe clubbing. Auscultation of the lungs revealed bilateral fine end inspiratory crepitation up to mid zone. Heart auscultation was normal. His peripheral oxygen saturation was 84% on room air.
Arterial blood gas revealed pH 7.45 PaO2 65 mmHg, PaCO2 30 mmHg. Pulmonary function test revealed FEV1/FVC 90.77, FVC 74%, FEV1 84%. His haemoglobin was 170g/L with normal white cell count, platelets, and biochemistry. Vasculitic screening was negative.
At this stage repeat CT chest showed bilateral progressive pulmonary infiltrates with thickening of the inter and intra-lobular septal lines and ground glass opacities or crazy-paving pattern. These changes were more confluent with slight lower lobe predilection and relative sub-pleural sparing. No overt bronchiectasis, discrete pulmonary nodules, masses, or cavitation seen (Figure 2).
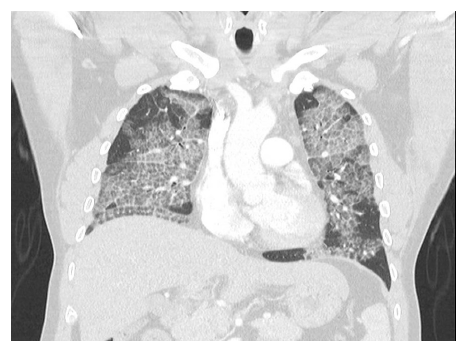
Figure2:Coronal view of CT done in 2021 showing crazy-paving pattern
Since crazy-paving pattern is not pathognomonic for PAP he was further investigated with bronchoscopic transbronchial cryo-biopsy which confirmed the diagnosis of auto immune PAP with positive anti-GM-CSF antibodies. During bronchoscopy, whitish gelatinous material noted endobronchially (Figure 3) and Broncho Alveolar Lavage (BAL) fluid showed the characteristic milky appearance. Cryo-biopsy of the lung confirmed the diagnosis of PAP with positive staining for PAS-D (Periodic Acid-Schiff with diastase) stain and negative staining for fungal infection (Figure 4). He was then referred for bilateral whole lung lavage.
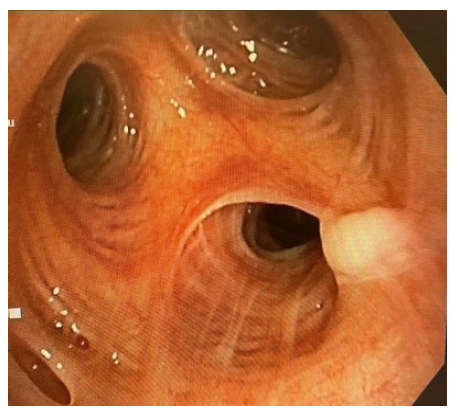
Figure3:Findings from bronchoscopy that showed accumulation of lipoproteinaceous material
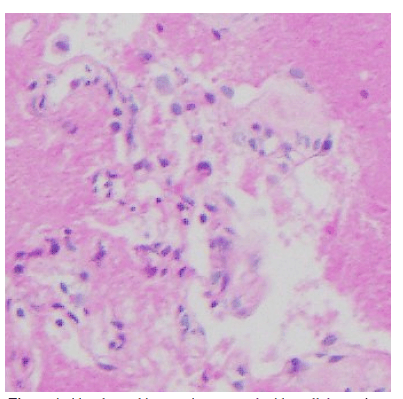
Figure4:Alveolar architecture is preserved with acellular eosinophilic granular material filling the alveolar spaces and highlighted with Periodic Acid-Schiff with diastase (PAS-D) stain. Foamy macrophages are not prominent
Discussion
PAP has a rare prevalence of 0.1-0.37 per 100,000 individuals [1, 3]. There are 3 types of PAP including congenital PAP, secondary PAP (due to inhalants, malignancies, or immunosuppression) and autoimmune PAP. More than 90 percent of the cases identified are autoimmune PAP, predominantly affecting middle-aged man around 40 to 50 years old.
Historically, autoimmune PAP was termed “idiopathic PAP” until the discovery of GM-CSF antibodies as the cause almost 40 years later [4, 5]. The development of PAP is thought to be related to reduction of granulocyte-macrophage colony-stimulating factor (GM-CSF) levels which is required for normal surfactant phospholipid production and clearance of protein by alveolar macrophage [6-8]. The GM-CSF receptors could be found on many cell lineages including macrophage precursors. GM-CSF plays role in terminal differentiation of monocytes to alveolar macrophage [8]. Importantly, the sensitivity and specificity of GM-CSF antibodies approached 100% for the diagnosis of autoimmune PAP [2].
The most common presentation of PAP would be insidious onset of dyspnoea. Nevertheless, one-third of patients will remain asymptomatic [1]. In terms of imaging studies, PAP patients will have the classic “bat-wing” appearance affecting mid and lower lung zones on Chest X-ray. High-resolution CT (HRCT) might show "Crazy-paving" pattern due to thickening of interlobular and intra-lobular septa [9]. It is seen less often in secondary causes of PAP although crazy-paving is not specific to PAP [9]. Common causes for crazy paving pattern other than PAP are acute respiratory distress syndrome, alveolar haemorrhage, cardiogenic pulmonary oedema, pneumocystis jirovecii pneumonia, bacterial pneumonia, adenocarcinoma of the lung [10]. One of the key steps to diagnosis of PAP is BAL via flexible bronchoscopy or transbronchial lung biopsy under fluoroscopy [11]. PAP is characterised by filling of pulmonary alveoli by periodic acid-Schiff (PAS) positive lipoproteinaceeous material [11].
The choice of treatment options for autoimmune PAP will based on the severity of the disease. More than 90% of the asymptomatic patients have a stable to improving course with only 8% worsening during follow up [12]. Therefore, supportive care including smoking cessation, have been recommended for patients with asymptomatic or mild disease [2]. For patients with moderate-to-severe disease will benefit from specific treatment [13]. Traditionally, the standard treatment of PAP has been whole lung lavage (WLL) since 1960s, where large quantities of saline are instilled into the lungs to physically remove the proteinaceous material. Therefore, a stepwise treatment approach starting with WLL, continuing to inhale GM-CSF followed by anti-CD20 monoclonal antibody i.e. Rituximab have been recommended if the former treatment regimens were unsuccessful [13].
Table 1.Demographics of patients with autoimmune PAP following cryptococcal meningitis with positive antibody
| Case | Age | Gender | Ethnicity | Initial presentations |
Subtypes of Cryptococcus |
Subsequent presentation post anti-fungal |
Extent of pulmonary involvement on CT |
Methods diagnose PAP |
Treatment following diagnosis PAP | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | M | Unspecified | Cryptococcal meningitis and cryptococcoma |
Cryptococcal gatii | Asymptomatic with radiological findings 3 years later | Bilateral ground glass opacities and crazy-paving pattern | Positive antibody and lung biopsy | Nil | Complete resolution of radiological changes and decrease in antibody titre |
| 2 | 20 | F | Unspecified | Meningoence phaltitis and left lower lobe cryptococcoma |
Cryptococcus neoformans | Asymptomatic with new radiological findings 2 years later. 1 year later, developed respiratory symptoms | Bilateral patchy scattered ground glass opacities | Positive antibody, BAL and lung biopsy | Initially then bilateral WLL | Improve in symptoms |
| 3 | 47 | M | Mexican | cryptococcoma complicated by meningitis | Cryptococcus neo formans | Asymptomatic with new radiological findings | Bilateral ground glass opacities |
Positive antibody and BAL | Nil | Ongoing monitoring |
| 4 | 44 | M | Bhutanese | Meningitis with possible cryptococcoma in left upper lobe | Cryptococcus neo formans Cryptococcal gatii | Respiratory symptoms with radiological findings | Bilateral ground glass opacities and crazy-paving pattern | Positive antibody, cryo-biopsy of the lung | Bilateral WLL | Ongoing monitoring |
BAL: Bronchoalveolar lavage; F:female; M:male; WLL:whole lung lavage
It is well established that autoimmune PAP is associated with opportunistic infections with Norcardia spp, mycobacteria, and fungal infection being the most reported pathogens. The mechanism of this association not precisely known and 40% of these patients will have the diagnosis of opportunistic infections preceding the diagnosis of PAP like in our case. However, the initial diagnosis of PAP may have been missed due to the rarity of the disease [14].
On the other hand, more recent publications have described invasive cryptococcal infection in seemingly immunocompetent patients [15, 16]. Interestingly, healthy individuals with anti-GM-CSF autoantibodies are more susceptible to meningoencephalitis caused by Cryptococcus, particularly with C. gattii [17, 18].
The presence of anti GM-CSF antibodies hinder the phagocytic properties of monocytes, macrophages and neutrophils which plays a major role in innate immunity against cryptococcal infection. There are in vivo and in vitro studies in the literature supporting above finding [19]. Furthermore, antibodies to GM-CSF can interfere with the action of specific proteins, STAT 5 (signal transducer and activator of transcription 5) and MIP-1 alpha (macrophage inflammatory protein -1 alpha) which are involved in phagocytosis and inflammatory reactions [19]. This can explain why the patients with anti-GM-CSF antibodies are more susceptible to opportunistic infection without underlying immunosuppression in addition to the development of PAP.
Patients and Methods
We report a case of autoimmune PAP with positive anti-GM-CSF antibodies that followed by cryptococcal meningitis and possible pulmonary cryptoccocoma needing whole lung lavage to control pulmonary symptoms. Although there were a few case reports that documented the association of cryptococcal infection with autoimmune PAP, many of these are reported pre-dated to the discovery of GM-CSF antibody as the link of autoimmune PAP to anti-GM-CSF antibody was only established in 2010 [20-22].
In terms of the method of testing, Immunoglobulin G (IgG) antibodies to GM-CSF were tested on serum using an ELISA binding recombinant human GM-CSF, where the results were reported in optical density units.
Literature Review
We conducted a comprehensive search of the English-language medical literature using Pub Med and the SA Health Library Service (SALUS) to identify articles reporting patients with autoimmune PAP following cryptococcal infection. The search terms include “Pulmonary alveolar proteinosis”, “PAP”, “autoimmune”, “acquired”, “Cryptococcus”, “Fungus”, “meningitis’, “Anti-GM-CSF”, “Granulocyte macrophage colony stimulating factor” and “antibody”.
There was one case report that described a patient who developed pulmonary cryptococcosis which later complicated by CNS cryptococcosis with positive GM-CSF antibody. Although there were CT chest finding that is consistent with early PAP, it was not mentioned whether the patient subsequently developed clinical PAP or diagnosis confirmed by bronchoscopy [23].
Based on our literature search, thus far, there is only one case report from France that reported a case of confirmed autoimmune PAP preceded by Cryptococcus gattii meningitis with positive anti-GM-CSF antibody [19]. Two similar cases were reported from America with Cryptococcus neoformans.
Case 1
Demir et al. reported a 42-year-old man with no history of immunosuppression developed cryptococcal meningitis and right para-hilar cryptococcoma due to Cryptococcal gatii. Antifungal treatment led to infection control and 3 years later CT chest revealed bilateral ground glass opacities and crazy-paving pattern without respiratory symptoms. Lung biopsy confirmed the diagnosis of PAP. Anti GM-CSF antibodies were positive in high titre. He was not offered any specific treatment and three years after the diagnosis of PAP patient remained asymptomatic with near complete resolution of radiological lesion and decrease in anti-GM-CSF antibody titre [19].
Another article from Rosen et al. reported seven healthy individuals who were diagnosed with cryptococcal meningitis with positive anti-GM-CSF antibody, and two patients later developed PAP in the following years [24].
Case 2
A 20-year-old HIV negative woman from California presented with Cryptococcus neoformans meningoencephalitis and a left lower lobe cryptococcoma. Her symptoms improved following antifungal medications. 2 years later CT showed resolution of the cryptococcoma but new bilateral patchy scattered ground glass opacities without pulmonary symptoms. BAL and transbronchial biopsies were consistent with PAP. As the patient was asymptomatic, she was not offered any specific treatment and opted for close observation. One year after the diagnosis of PAP she re-presented with progressive CT changes and respiratory symptoms. Bilateral whole lung lavage improved her symptoms.
Case 3
Previously healthy HIV negative 47-year-old Mexican man from California presented with biopsy confirmed large perihilar mass due to Cryptococcus neoformans and one week later developed cryptococcal meningitis. Pulmonary and CNS infections improved with antifungal medications, 4 years later represented with new progressive bilateral ground glass opacities without pulmonary symptoms. BAL showed PAS positive material and confirmed PAP. Since he was asymptomatic no therapeutic intervention has been performed. The patient was for ongoing surveillance without intervention at the time of publication.
We believe this could be the fourth published case with similar antecedent event prior to the diagnosis of autoimmune PAP and the second such patient who required whole lung lavage for symptom control. Unlike the previous case, our patient was diagnosed with cryptococcal meningitis with two different types of cryptococci at the same time.
It could be argued that our patient too developed cryptococcal meningitis and autoimmune PAP due to the presence of anti-GM-CSF antibodies which was not measured when the initial diagnosis of meningitis. The alternative hypothesis that the development of PAP is a sequela of the cryptococcal infection triggering autoimmunity. Therefore, our study would also like to emphasise the importance of checking anti-GM-CSF antibody in any otherwise healthy individual with diagnosis of cryptococcal meningitis. Only certain percentage of patients with anti-GM-CSF antibodies associated cryptococcal infection will develop autoimmune PAP, which the cause is still unclear. Therefore, we could not predict the likelihood of development of PAP and severity of disease based on the presence of antibody alone due to the paucity of available data. Our patient had severe lung involvement with hypoxemia requiring whole lung lavage. However, according to the literature some patients can be managed without specific intervention. Nevertheless, close observation is warranted to early identification of disease progression.
Conclusion
In summary, diagnosis of opportunistic infection might precede the diagnosis of PAP in immunocompetent patients and we urge all clinicians to be more vigilant especially in patients with radiological findings. Anti-GM-CSF antibodies should be measured in any healthy individuals that develop cryptococcal meningitis to expand the current database to facilitate prediction of PAP development in future.
Acknowledgement
We would like to thank pathologist, Dr Sean Chang for providing the pathology slides for publication. No funding was involved in this publication.
References
1. Rosen SH, Castleman B, Lieobow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958; 258 (23): 1123-1142.
2. Suzuki T, Trapnell B. Pulmonary alveolar proteinosis syndrome. N Engl J Med. 2016; 37(3): 431-440.
3. Ben-Dov I, Kishinevski Y, Roanman J, et al. Pulmonary alveolar proteinosis in Israel: ethnic clustering. Isr Med Assoc J. 1999; 1(2):75-78.
4. Kitamura T, Tanaka N, Watanabe J, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999; 190(6): 875–880.
5. Uchida K, Nakata K, Trapnell BC, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004; 103 (3): 1089–1098.
6. Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol. 2010; 135 (2): 223-235.
7. Seymour JF, Doyle IR, Nakata K, et al. Relationship of anti-GM-CSF antibody concentration, surfactant protein A and B levels, and serum LDH to pulmonary parameters and response to GM-CSF therapy in patients with idiopathic alveolar proteinosis. Thorax. 2003; 58(3): 252-257.
8. Browne SK, Holland SM. Anticytokine autoantibodies in infectious diseases: pathogenesis and mechanisms. Lancet Infect Dis. 2010; 10 (12): 875-885.
9. Claypool WD, Rogers RM, Matuschak GM. Update on the clinical diagnosis, management, and pathogenesis of pulmonary alveolar proteinosis (phospholipidosis). Chest. 1984;85 (4): 550-558.
10. Rossi SE, Erasmus JJ, Volpacchio M, et al. "Crazy-paving" pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23(6):1509-1519.
11. Bonella F, Bauer PC, Griese M, et al. Pulmonary alveolar proteinosis: new insights from a single-center cohort of 70 patients. Respir Med. 2011;105 (12):1908–1916.
12. Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008 Apr 01;177(7):752-762.
13. Leth S, Bendstrup E, Vestergaard H, et al. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology. 2013;18(1):82–91.
14. Punatar AD, Kusne S, Blair JE, et al. Opportunistic infections in patients with pulmonary alveolar proteinosis. J Infect. 2012; 65(2):173–179.
15. May RC, Stone NR, Wiesner DL, et al. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016;14 (2): 106-117.
16. Chen S, Sorrell T, Nimmo G, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 2000; 31(2): 499-508.
17. Kwon-Chung KJ, Sajio T. Is Cryptococcus gattii a primary pathogen? J Fungi (Basel). 2015; 1(2): 154-167.
18. Saijo T, Chen J, Chen SC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014; 5(2). E00912-00914.
19. Demir S, Chebib N, Thivolet-Bejui F, et al. Pulmonary alveolar proteinosis following cryptococcal meningitis: a possible cause? BMJ Case Rep. 2018.
20. Browne SK, Holland SM. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol. 2010;10(6):534–541.
21. Bergman F, Linell F. Cryptococcosis as a cause of pulmonary alveolar proteinosis. Acta Pathol Microbiol Scand. 1961; 53:217-224.
22. Sunderland WA, Campbell RA, Edwards MJ. Pulmonary alveolar proteinosis and pulmonary cryptococcosis in an adolescent boy. J Pediatr. 1972; 80 (3): 450-456.
23. Stevenson B, Bundell C, Mulrennan S, et al. The significance of anti-granulocyte-macrophage-colony-stimulating factor antibodies in cryptococcal infection: case series and review of antibody testing. Int Med J. 2019; 49: 1446-1450
24. Rosen LB, Freeman AF, Yang LM, et al. Anti–GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013; 190(8):3959-3966.
Received: July 23, 2021.
Accepted: August 10, 2021.
Published: August 12, 2021.
To cite this article : Lim WT, Priyangika SMTN, Karunarathne JSKP. Crazy-Paving Pattern: A Rare Case of Autoimmune Pulmonary Alveolar Proteinosis (PAP) with Positive Anti-GM-CSF Antibody Following Cryptococcal Infection in an otherwise Healthy Individual and Review of Literature. European Journal of Respiratory Medicine. 2021; 3:2.
© 2021 Lim WT, et al.
