Review article / Open Access
DOI: 10.31488/ejrm.106
Cystic Fibrosis Therapy: From Chest Physiotherapy to Agents Targeting Specific Mutations
Janice Wang*1,Rubin I Cohen*2
1. Director ICU, Salisbury VA Medical Center Professor of Clinical Medicine, Texas TechSchool of Medicine, USA
*Corresponding author:Rubin I Cohen, Director ICU, Salisbury VA Medical Center Professor of Clinical Medicine, Texas Tech School of Medicine, USA
Introduction
Cystic Fibrosis (CF) is an autosomal recessive genetic disorder that was a terminal illness in young children. Standardized care in accredited CF Centers and medical advances in maintenance medications targeting nutritional health, CF bacterial pathogens, inflammation, mucociliary clearance, and the underlying defect in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, improved life expectancy and quality of life for people with CF[1,2]. Today, half of the United States CF population is of adult age. According to the 2018 Cystic Fibrosis Foundation (CFF) Patient Registry, 54.6% were 18 years and older[3]. The life expectancy of people with CF born between 2014 and 2018 is predicted to be 44.4 years (95 percent confidence interval: 43.4–45.9 years) [3,4].
The discovery of the CFTR gene in1989triggered aa surge in basic research[5].While this enhanced knowledge of the pathophysiology of CF and clarified genotype-phenotype relationships, it was not until 2012 that an agent targeted towards correcting the defective protein became a reality [6].Until then, theavailable therapies could only improve symptoms and slow down the progression of the disease.Nevertheless, such therapies transformed CF from a fatal disease of children to a more treatable, albeit still fatal,chronic disease of adults [7].
The most recent and swiftly evolving therapies in CFtherapeutic pipeline are the CFTR modulator targeting the abnormal protein. The CFTR protein is a cAMP protein kinase chloride ion channel that regulates chloride and bicarbonate transportacross the epithelial cell surface and, in turn, inhibits the absorption of sodium. CFTR protein is encoded by the CF gene located on chromosome 7 (7q31.2) and is found in epithelial cells of the airway, intestine, pancreas, and sweat gland. In CF, the CFTR protein defect is dependent on the Class of CFTR mutations, and total CFTR activity is determined by the CFTR quantity and function [8]. There are six classes of CFTR mutations (Table 1).
| Mutation Class |
Protein Defect | Mutation Examples |
|---|---|---|
| Ⅰ | Completely absent due to nonsense or frame shift mutation | G542X, W1282X, R553X |
| Ⅱ | Misfoldedprotein does not reach cell surface | F508del, N1303K |
| Ⅲ | Protein at cell surface but has decreased activity | G551D, S549N, V520F |
| Ⅳ | Protein at cell surface but chloride conductance is reduced | R117H, D1152H, R347P |
| Ⅴ | Quantity of protein is reduced | A455E, 3849 + 10kc -> T |
| Ⅵ | Decreased protein stability | 4326deITC, Gln1412X |
The full explanation of CF airway pathology is beyond this review.Briefly, normal cilia require a hydrated airway surface liquid layer to function appropriately in mucociliary clearance. When chloride ion transport is impaired or absent based on the type of CFTR mutation, sodium is hyper-absorbed along with water, resulting in a poorly hydrated airway surface liquid layer. This leads to a continual cycle of thick mucus, airway obstruction and inflammation, tissue injury and resultant bronchiectasis. Once bronchiectatic airways develop, airway clearance is not optimal.This environment promotes the acquisition of bacteria that become pathogenic in CF lungs[9,10].
This article summarizes the available treatments for CF categorized by the mechanism of action.Webegin by explaining traditional CF therapies and go on to describe CFTR modulator therapy in more detail.
Airway Clearance Therapies
Airway clearance remains a mainstay of CF therapy and is encouraged from birth to adulthood [10]. However, since many clinical trials assessing airway clearance therapies were not adequately powered and often not randomized, definitive evidence supporting the use of airway clearance therapies in CF is lacking. The recommendations to use airway clearance therapies in CF rely instead on sound theoretical principles [11]. Techniques include percussion, device assistance including vibrating vest, flutter, positive expiratory pressure, and breathing modalities (autogenic drainage). One study compared the conventional active cycle of breathing technique, routinely practiced in many countries, to the more costly, albeit more convenient, vibrating vest.The active cycle modality was superior to the vest and associated with fewer pulmonary exacerbations [12].
Mucolyticsand Hydrator Therapies
Human recombinant deoxyribonuclease was the first approved therapy for CF. This nebulized medication decreases sputum viscosity, improves lung function, and reduces exacerbations.It works by breaking down DNA derived from degrading neutrophils that accumulate in the airways of CF patients [13]. Further studies demonstrated benefits in CF patients with advanced lung disease (FEV1 less than 40% predicted) and in younger patients with mild disease [11].
Nebulized hypertonic saline behaves as an osmotic hydrating agent that increases mucociliaryclearance, improves lung function, and reduces exacerbations. In a 48‐week study, 164 CF patients were randomized to receive either 5 ml of 7% hypertonic saline or placebo [0.9% saline] with quinine sulfate added to mask the taste. FEV1 was higher, and pulmonary exacerbations were fewer in the hypertonic saline group[14].While subsequent studies did not observe a reduction in exacerbations in children aged four months to 5 years, sub-studies demonstrated positive effects on lung function [11].
Inhaled dry powder mannitol is another osmotic agent that demonstrated lung function improvement in two trials with the effects being more consistent in adults[15].The drug was first approved in Australia in 2011, subsequently in the EU and Israel. In May 2019, the US-based Federal Drug Administration [FDA] advisory panel recommended approval.Concerns include a high dropout rate since some patients could not tolerate inhaled mannitol[11].Subsequent trials stressed education and training to achieve adequate treatment adherence [16].Before starting mannitol, patients must complete the mannitol tolerance test [sequential administrations of incremental doses of mannitol up to a maximum dose of 400 mg] to identify those at risk for mannitol-induced bronchospasm[16].Those who develop bronchospasm should not use mannitol.
Inhaled Antibiotics
Inhaled antibiotics offer advantages over systemic therapy since they deliver high drug concentrations to the site of lung infection with minimal systemic absorption[17]. In patients with chronic P. aeruginosa infection, nebulized tobramycin (an aminoglycoside) improved lung function, reduced exacerbations, and increased weight[18].Nebulized tobramycin [tobramycin inhaled solution or TIS] was first approved in 1997 and subsequently manufactured by other drugmakers when its patent expired.Inhaled aztreonam (a β‑lactam) is effective when compared with both placebo and inhaled tobramycin and was approved in 2010 [19,20].
Sixteen years following the deployment of TIS, a dry powder preparation became available. This new preparation substantially decreases delivery time, is more portable, and does not require refrigeration [21].
To thwart the potential emergence of resistant bacteria, the protocols for inhaled antipseudomonal antibiotics in CF called for every other month administration: 28 days “on” therapy, alternating with 28 days “off” therapy.The problem with this approach is that during the “off” periods, lung function deteriorates, and patients report lower quality of life [22]. Consequently, many CF physicians shifted to continuous inhaled antibiotic therapy, either as monthly monotherapy or as continuous alternating therapy (CAT) with two or more agents [22].
Flume and colleagues [23], conducted a randomized, prospective trial to determine whether a continuous antibiotic regimen offers further improvement in pulmonary outcomes compared with the intermittent strategy.Unfortunately, the study experienced limited enrolment since practice patterns had already changed with CAT becoming routine in many CF centers.Even though this study was underpowered, CAT -one month of nebulized aztreonam alternating with one month of dry inhaled tobramycin- reduced exacerbation rates by 25.7% (p = 0.25; primary endpoint) and rates of respiratory hospitalizations by 35.8% compared with dry inhaled tobramycin alone (p = 0.14).
Amikacin liposome inhalation (ALI) is only approved to treat chronic lung infections caused by nontuberculous mycobacteria (NTM).ALI is approved in adults in combination with an anti-NTM drug regimen. Patients should have documented failure/inadequate response after at least six consecutive months of an anti-NTM multidrug regimen. ALI is administered via a specific nebulizer once daily at a dose of 590 mg[23].
As far as ALI for pseudomonal infection in CF, a phase II trial demonstrated once-daily ALI tolerability, safety, biologic activity, and efficacy in CF patients with P aeruginosa infection [24].A recent study compared ALI to tobramycin inhaled solution and found a comparable effect on FEV1.ALI patients demonstrated improvement in CFQ-R respiratory symptoms.Furthermore, subjects reported less treatment burden in the ALI arm [Once daily ALI compared to twice daily tobramycin].Furthermore, there was increased biofilm penetration with ALI when compared to tobramycin [25].Thus far, ALI is not approved for pseudomonal CF infections.
Other antibiotics such as nebulized colistin[Colisteneb] and colistimethate sodium dry powder inhaler [Colobreath] have received European approval.Several new preparations of inhaled antibiotics are under study[11,26]. Specifically, dry powder vancomycin for the increasing rate of MRSA chronic infections -presently, about 20% of the US CF population- demonstrated a significant reduction in MRSA density in the sputum compared with placebo[26].A phase 3 study to test AeroVanc™ in adults and children over six years old with CF is underway [AVAIL NCT03181932]. Few studies have so far examined the role of inhaled antibiotics for other bacterial infections that are common in patients with cystic fibrosis (B. cepacia complex and S. maltophilia[26].
Pseudomonas Eradication Therapy
Once Pseudomonalchronic infection is established, it is virtually impossible to eradicate and is associated with increased mortality and morbidity [10,11,17,27,28]. At the time of the first culture of P aeruginosa, CF physicians prescribe antibiotic treatment with the aim of eradicating.A Cochrane review concluded that nebulized antibiotics, alone or in combination with oral antibiotics, were better than no treatment for early infection with P.aeruginosa and that eradication may be sustained for up to two years.A regimen of oral ciprofloxacin and inhaled colistin for three months has been prescribed based on evidence from a prospective study that used historical control data. Further studies demonstrated that inhaled tobramycin, either TIS or in dry powder form, for one month eradicates P.aeruginosa in 70–80% of cases.More importantly, this regimen is equivalent to the ciprofloxacin plus colistin combination [28].Experts recommend both regimens for Pseudomonas eradication therapy.Ensuring eradication of P aeruginosa is routine in CF care, with many pediatric centers reposting a prevalence of chronic P aeruginosa of less than 10% [11].
Azithromycin
Macrolides exhibit bacteriostatic activity by inhibiting protein synthesis through disturbance of the 50S large ribosomal subunit and subsequent interruption of bacterial protein synthesis. Relevant to CF, azithromycin reduces biofilm growth of bacteria such as Pseudomonas aeruginosa by impairing quorum-sensing signals. Additionally, azithromycin increases P. aeruginosa susceptibility to other antibiotics[29].Moreover, azithromycin possesses well-documented immunomodulating properties. These include an acute phase of inhibition of inflammation and a late phase of the resolution of chronic inflammation reviewed in[30].
To determine whether azithromycin improves clinical parameters and reduces inflammation in CF adults, Wolter, and colleagues conducted a 3-month prospective randomized, double-blind, placebo-controlled study of 250 mg of daily azithromycin[31].This study recruited 60 subjects and concluded that azithromycin improved quality of life, reduced the number of respiratory exacerbations, and the rate of decline in lung function. Saiman and colleagues conducted a more extensive, 24-week study that randomized 185 participants, all chronically infected with Pseudomonas.The group that received 500 mg of azithromycin thrice weekly showed an increase in FEV1, had a more substantial weight gain while experiencing fewer exacerbations[32].
A subsequent study sought to determine the effects of azithromycin in CF patients uninfected with Pseudomonas. In this trial, treatment with azithromycin for 24 weeks did not result in improved pulmonary function. Nevertheless, azithromycin was associated with a reduction in pulmonary exacerbations and an increase in weight gain [33].
As would be expected, bacterial resistance increases with chronic antibiotic use. A meta-analysis of six trials using azithromycin therapy in chronic lung diseases such as asthma, COPD, and CF, concluded that the risk of bacterial resistance was 2.6 times higher in the treatment group (RR = 2.59; 95% CI: 1.294–5.31; p = 0.007)[29,30].Of concern in the CF population, is the emergence of nontuberculous mycobacterial infections. Therefore, CF centers routinely screen for such infections before starting and during chronic azithromycin therapy.
Further concerns about the chronic use of azithromycin in CF revolve around its potential antagonism of inhaled tobramycin.An in vitro model demonstrated that azithromycin upregulated the MexXY efflux pump of P. aeruginosa, reducing the aminoglycoside concentration within the bacterial cell [34].
A secondary analysis of a clinical trial of the inhaled antipseudomonal antibiotics tobramycin, with and without concomitant azithromycin concluded that combined azithromycin and inhaled tobramycin resulted in a decrease in % predicted FEV1 after one and three courses of inhaled tobramycin when compared with those not using azithromycin (28 d: 20.51 vs. 3.43%, P, 0.01; 140 d: 21.87 vs. 6.07%, P, 0.01).Additionally, chronic azithromycin with inhaled tobramycin -and not inhaled aztreonam- was associated with an earlier need for additional antibiotics, smaller improvement in the quality of life, and a trend toward less reduction in sputum P. aeruginosa density [35].
On the other hand, the Optimizing Treatment for Early Pseudomonas aeruginosa Infection in Cystic Fibrosis (OPTIMIZE) sought to improve early eradication of P. aeruginosa.The investigators hypothesized that adding azithromycin (vs. placebo) to the inhaled tobramycin would reduce the risk of pulmonary exacerbation, inflammation and could prolong the time to pseudomonas recurrence.While adding azithromycin to inhaled tobramycin did not increase eradication rates among children receiving azithromycin, there was a 44% reduction in pulmonary exacerbations, a 1.3 kg weight gain, and no changes in bacterial milieu. These findings suggest that the addition of azithromycin did not interactwith tobramycin[36].
An ongoing clinical trial may untangle potential antagonism between inhaled tobramycin and chronic oral azithromycin in CF: Testing the Effect of Adding Chronic Azithromycin to Inhaled Tobramycin (TEACH; NCT02677701), randomizes participants to receive inhaled tobramycin with azithromycin vs. placebo[29].
Anti-Infective Therapies
In an era of multidrug-resistant bacteria, research focuses on non-antibiotic, anti-infective therapies, including phage therapy, inhaled nitric oxide, and IV gallium. Bacteriophages or phages are viruses found in the environment.They invade and replicate a hundred-fold within targeted bacteria, ultimately bursting the host bacterial cell. Technological advances allow for the creation of viruses with more accuracy to their bacterial targets. For example, phages have been modified for nebulization against P.aeruginosa, Mycobacterium abscessus, and Achromobacter[37].A case report describes a 15-year old CF patient with a double-lung transplant who received a genetically engineered intravenous and topical 3-phage regimen against M. abscessus, which helped wound healing and improved lung function [38].
The metal gallium is postulated to inhibit iron‐dependent bacterial enzymes by impeding many iron-dependent synthetic and metabolic pathways[39].The advantages to gallium are that the IV form is already FDA-approved for the treatment of malignancy-associatedhypercalcemia, and gallium has been used for decades in radiological studies.Goss et al. enrolled 20 CF adults chronically infected with P.aeruginosa, who received IV gallium. Lung function improved, and this improvement persisted until day 28. Bacterial density, however, was not significantly decreased. Moreover, the IV form of gallium involves a 5-day continuous infusion, making it quite inconvenient for patients[39].AR-501 is an inhalable form of gallium citrate; safety and pharmacokinetic study of inhaled gallium are underway (NCT03669614).
The antimicrobial activity of nitric oxide (NO) had been recognized for decades [40].In a twelve-patient randomized proof of concept study, Howlin and colleagues [41],used an ex-vivo model and studied 12 patients randomized to receive ten ppm NO inhalation or placebo.They found a significant reduction in P. aeruginosa biofilm aggregates compared with placebo across seven days of treatment. Other measurements of clinical parameters favored NO without an increase in overall bacterial load or the severity of acute exacerbations. There was no evidence of NO-induced vasodilatation. Additionally, these investigators demonstrated enhanced in vitro activity of tobramycin and tobramycin plus ceftazidime when combined with NO. An ongoing phase II clinical trial of inhaled NO is underway (NCT02498535).
Anti-Inflammatories
Studies investigating immune cell function in CF overwhelmingly concluded that the CF inflammatory response is abnormal and hyperinflammatory.Furthermore, the pulmonary inflammation observed in CF may not be solely the result of chronic infection and mayoccur in the absence of prior infection (26). While the relationship between abnormal CFTR function and inflammation is not fully understood, evidence points to the CFTR mutation in airway epithelial cells as either instigating or at least contributing to the CF inflammatory response. It is yet undetermined whether CFTRpotentiators and correctors, and the ensuing improved CFTR function will translate into downstream improvements in inflammatory profiles[42].
Investigators have long established that neutrophils have the highest accumulation density of any inflammatory cell in the lungs of CF patients. Even though an enormous number of neutrophils are recruited to the CF lungs during infection, this does not result in an increased ability to clear the infection; instead, it leads to hyperinflammation and tissue injury. Subsequently, in the CF airway, chronic inflammation leads to chronic obstruction and permanent destruction and dilation of the airway.Thus, anti-inflammatory drugs are attractive agents to modify this chronic inflammation and preserve lung function in CF[42].
Earlier studies examined the effects of corticosteroids (CS) in CF children. Results from the first trial were reported in 1985. CF patients age 1 to 12 years with mild to moderate lung disease who received alternate-day oral prednisone (2 mg/kg) had better pulmonary function and growth and fewer hospitalizations.However, growth retardation, glucose intolerance, osteoporosis, and cataracts were frequent.In 1995, a much larger multicenter trial compared two alternate- day dosing regimens (2 mg/kg and 1 mg/kg) with placebo over four years in 285 CF patients, aged 6 through 14 years. Although there were beneficial effects on lung function, notably in those chronically infected with Pseudomonas, growth retardation, glucose abnormalities, and cataracts were frequent even with the 1 mg/kg dose[43].Because of adverse effects, the CF Foundation does not recommend the chronic use of oral CS to improve lung function in CF children. In adults with CF, oral CS have not been shown to improve lung function.Since CF is now a chronic disease of adults, there is a genuine concern that the prolonged use of systemic CS would lead to the development of steroid-related diabetes mellitus, earlier development of CF-related diabetes mellitus [CFRD] and osteoporosis.
Inhaled Corticosteroids
Clinical trials did not show a benefit to lung function or reduction in hospitalizations with the routine use of inhaled corticosteroids [ICS]. A Cochrane Review of ICS determined that there is not enough evidence to support the routine use of ICS[44].The CF Foundation recommends against the routine use of ICS in CF without asthma.
Ibuprofen
Ibuprofen, with its specific activity against neutrophils, is an attractive agent in CF. It is also an inhibitor of cyclo-oxygenase that converts arachidonic acid into prostaglandins and thromboxanes. Oral doses of ibuprofen that result in a high plasma concentration (> 50 mg/ml) were associated with a decreased neutrophil migration to the mucosal epithelia and a slower decline in FEV1, especially in younger patients [21,45,46].Nevertheless, ibuprofen has not been widely adopted, primarily because of the challenges associated with establishing such high plasma levels, difficulty in routinely measuring levels, and potential adverse effects, including gastrointestinal bleeding and kidney injury.
Novel anti-inflammatories under investigation
Lenabasum (JBT-101) is an oral anti-inflammatory drug that promotes the resolution of inflammation and fibrotic responses by binding to and activating the cannabinoid receptor type 2 on immune cells. Preliminary studies in CF showed that this drug was safe and well-tolerated. There were trends towards a reduction in inflammation and reduced pulmonary exacerbations. A phase IIbmulticentre, double-blind, randomized, placebo-controlled study assessing the efficacy and safety of lenabasum for the treatment of CF 12 years of age or older is underway[47].
Acebilustat (CTX-4430) is a once-daily oral inhibitor of LTA4 hydrolase that prevents the formation of LTB4, a potent neutrophil chemoattractant. In a 200-patient study, [EMPIRE-CF] once-daily acebilustat reduced the frequency of exacerbations by 19% over 48 weeks in CF patients, regardless of genotype. However, the investigators did not observe any difference in lung function[48].A phase III study is planned.
CFTRModulators
There are four FDA- approved CFTR modulators that demonstrate enhanced functional activity of the abnormal CFTR protein [Figure 1]. Vertex Pharmaceuticalsis the manufacturer [2,6,7,9,49].
Ivacaftor (VX770; Kalydeco®)
Ivacaftor (IVA) is a CFTR potentiator that increases the open probability of the CFTR channel resulting in augmented chloride transport. It is the first FDA-approved CFTR modulator for those six years and older with CF mutation G551D. In a randomized, double-blind, placebo-controlled trial, a total of 161CF patients 12 years of age or older with at least one G551D mutation were randomly assigned to receive 150 mg of IVA every 12 hours orplacebo for 48 weeks[50].Baseline mean %predicted FEV1 was 63.6.Improvement in %predicted FEV1and reduction in pulmonary exacerbationrate was observed in the IVA group compared to the placebo group by week 24 [Figure 1]
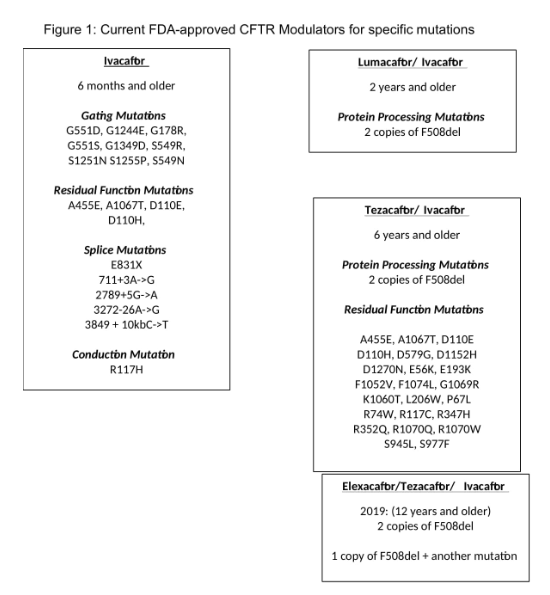
In the post-approval GOAL study on patients six months after IVA initiation, sweat chloride values were decreased to a mean value of 49 (P<0.001) [51]. Body mass index (BMI) also improved by an average of 0.8 kg/m2 (P<0.001).
Subsequently, IVA was approved for CF mutations that produce a CFTR protein with residual function [Classes IV and V] based on in vivo and in vitro data [52-54].
Lumacaftor-Iva (VX809-VX770; Orkambi®)
Lumacaftor (LUM) isa CFTR corrector that corrects Phe508del CFTR misprocessing and increases the amount of cell surface-localized protein.
Two-phase 3, randomized, double-blind, placebo-controlled studies, TRAFFIC, and TRANSPORT assessed the effects of LUM combined with IVA at different dosages for 24 weeks in CF patients ages 12 years and over who were homozygous for the Phe508del CFTR mutation[55].Study design and data analysis were identical, apart from TRAFFIC, including ECG assessments and TRANSPORT, incorporatingadolescent pharmacokinetic assessments. A total of 1,108 patients were randomly assigned to one of three groups: (1) placebo; (2) LUM 600 mg once daily plus IVA 250 mg every 12 hours; and (3) LUM 400 mg every 12 hours plus IVA 250 mg every 12 hours. Pooled analyses from both studies for the subgroup LUM 400 mg/IVA 250 mg every 12 hours improved %predicted FEV1 by a mean of 2.8 points (P<0.001). Pulmonary exacerbations rate was 30 to 39% lower in the LUM/IVA groups than in the placebo group.The rate of pulmonary exacerbations leading to hospitalization and the use of intravenous antibiotics through week 24 in the LUM/IVA group was reduced by 61% (P < 0.001) and by 56% (P < 0.001), respectively when compared to the placebo group.
Pulmonary exacerbations were fewer in the LUM (400 mg every 12 h)/IVA group (35.8%), favoring this dose over the LUM 600 mg daily/IVA (39.3% patients with pulmonary exacerbations). The most common adverse events were mild to moderate in severity and included pulmonary exacerbations, cough, headache, dyspnea, and hemoptysis. Respiratory events occurred within 1 to 2 days after starting therapy and resolved within the first 2 to 3 weeks. The rate of discontinuation due to adverse events was 4.2% in the LUM/IVA group versus 1.6% in the placebo group.
Elborn et al. examined the subpopulations of CF patients who developed a decline in %predicted FEV1 of < 40 % during the TRAFFIC and TRANSPORT trials and found improvement in %predicted FEV1 compared to placebo.However, there were more respiratory adverse events in the more advanced FEV1 subgroup [56].Caution is therefore advised when patients with %predicted FEV1< 40% are prescribed LUM/IVA.
An extension study over an additional 72 weeks of treatment was conducted to assess the safety and efficacy of long-term with LUM/IVA.PROGRESS enrolled 1,030 patients from the TRANSPORT and TRAFFIC in phase 3, parallel-group, study [57].LUM (400mg every 12 h) combined with IVA (250 mg every 12 h)was associated with a 42% slower rate of %predicted FEV1 decline compared to matched registry controls. The annualizedpulmonary exacerbationrate through extension week 96 in TRAFFIC (0.65) and TRANSPORT (0.56) was lower than that in the placebo group (0.75). The safety profile of LUM/IVA was the same as the original trials.
Albeit transient, side effects of LUM such as worsening cough, chest discomfort, and dyspnea led some CF patients, especially those with more advanced disease, to discontinue the drug.Afew CF providers questioned whether the aggravated cough and dyspnea justified the potential benefit of a 2.8% improvement in FEV1 that was statistically significant, but was it clinically meaningful? Additionally, LUM is a potent inducer of CYP3A, while IVA is a CYP3A-sensitive substrate. The combination could impact on medications that are substrates of CYP3A by modifying their effect.Despite its side effects and potential drug interaction, LUM/IVAtargeted those with the most common CF mutation [detaF508] and loweredthe incidence of acute pulmonary exacerbations[58].CF physicians prescribed LUM/IVA in the hope that it would further impede disease progression and maintain lung health. Nevertheless, the CF community awaited the development of a better agent.This was Tezacaftor [TEZ].
Tezacaftor-IVA (VX661-VX770; Symdeko®)
TEZ is a CFTR corrector that improves cellular processing and trafficking of the CFTR protein, thereby increasing the quantity of CFTR at the cell surface and resulting in increased chloride transport. TEZ has fewer respiratory side effects than LUM and TEZ is not an inducer of CYP3A, thus reducing the potential for drug-drug interactions
The combination of TEZ/IVA was approved for those with homozygous F508del mutation or heterozygous for one of 26 mutations based on the EVOLVE and EXPAND trials. EVOLVEwas a phase 3, randomized, double-blind, placebo-controlled, parallel-group trial evaluating the efficacy and safety of TEZ/IVA in patients 12 years and older with CF who were homozygous for the Phe508del CFTR mutation[59].Patients were randomly assigned to receive either placebo or treatment consisting of a combination tablet of TEZ 100 mg/IVA 150 mg once daily in the morningand IVA150 mg once daily in the evening for 24 weeks. The mean absolute change from baseline was 3.4 percentage points in the TEZ/IVA group and -0.6 percentage points in the placebo group, a difference of + 4.0 points in %predicted FEV1 from baseline between TEZ/IVA and placebo groups (P <0.001). The pulmonary exacerbation rate in the TEZ/IVA group was 35% lower compared to the placebo group.Serious adverse events occurred less frequently in the TEZ/IVA group compared to placebo.
The EXPAND trial(51) randomized patients(51) to receive two 8-week intervention periods separated by a washout period of 8 weeks. Interventions included: (1) TEZ 100 mg once daily/IVA 150 mg every 12 h; (2) IVA 150 mg every 12 h; (3) placebo. Compared to placebo, the mean difference in absolute change in %predicted FEV1 for TEZ/IVA and IVA alone was 6.8 and 4.7 percentage points, respectively (P < 0.001). Similar to the EVOLVE trial, most adverse events were mild to moderate.
Elexacaftor-Tezacaftor-IVA (VX445-VX661-VX770; Trikafta®)
Elexacaftor (ELX) is a “next-generation” corrector since it has a different structure and mechanism when compared to first-generation correctors such as LUM and TEZ.The concept is to combine ELX with TEZ to further increase CFTR protein processing and trafficking to the cell surface.IVA is added to this combination to improve potentiation and chloride channel opening[60].
Patients were randomized to the combination of ELX 200 mg once daily/TEZ 100 mg once daily/IVA 150 mg every 12 hours or placebo. Compared to placebo, %predicted FEV1 improved by 13.8 points at four weeks and 14.3 points by week 24. The rate of pulmonary exacerbations was 63% lower than the placebo. The triple combination drug had an acceptable safety profile.
CF patients homozygous for the Phe508del mutation were enrolled in the triple ELX/TEZ/IVA combination.All were started in a 4-week TEZ/IVA run-in period, followed by randomization to 4 weeks of ELX 200 mg once daily/TEZ 100 mg once daily/IVA 150 mg every 12 hours OR TEZ 100 mg once daily/IVA 150 mg every 12 hours[61].Compared to the TEZ/IVA group, the ELX/TEZ/IVA group had a 10-point increase in %predicted FEV1 (Table 2).
| Ivacaftor 150 mg twice daily |
G551D | 10.6 higher P < 0.001 |
55% lower P < 0.001 |
0.8 kg/m2 | 8.6 points higher P <0.001 |
2.7 kg higher P < 0.001 |
48.1 mmol per liter lower P < 0.001 |
NA | NA |
|---|---|---|---|---|---|---|---|---|---|
| CFTR Modulator |
Targeted Genotype |
Week 24 Absolute change from baseline in ppFEV1 vs. placebo |
Week 24 Reduction in Pex vs. placebo |
Week 24 bsolute change from baseline in BMI vs. placebo |
Week 24 Absolute change from baseline in Respiratory domain of CFQ-R vs. placebo |
Week 48 Absolute change from baseline in weight gain vs. placebo |
Week 48 Absolute change from baseline in Sweat chloride vs. placebo |
Week 96 Absolute change from baseline BMI |
Week 96 Change from baseline Respiratory domain of CFQ-R |
| Lumacaftor- Ivacaftor TRAFFIC/ TRANSPORTa |
Homozygous Phe508del |
2.8 higher P < 0.001 |
39% lower P <0.001 |
+ 0.24 P < 0.001 |
2.2 points higher P = 0.05 |
NA | NA | NA | NA |
| Lumacaftor- Ivacaftor PROGRESSb |
Homozygous Phe508del |
NA | NA | NA | NA | NA | NA | + 0.96 P = 0.4231 |
+ 3.5 P = 0.0018 |
| Tezacaftor- Ivacaftor EVOLVE |
Homozygous Phe508del |
4.0 higher P < 0.001 |
35% lower P = 0.005 |
+ 0.06 | 10.1 mmol per liter lower | ||||
| Absolute change from baseline in ppFEV1 vs. placebo |
Change in Respiratory domain of CFQ-R vs. placebo |
||||||||
| Tezacaftor -Ivacaftor EXPANDc |
Residual- Function Heterozygotes |
TEZ/IVA 6.8 higher P < 0.001 |
IVA 4.7 higher P < 0.001 |
TEZ/IVA 11.1 higher P < 0.001 |
IVA 9.7 higher P < 0.001 |
||||
| CFTR Modulator |
Targeted Genotype |
Week 24 Absolute change from baseline in ppFEV1 vs. placebo |
Week 24 Reduction in Pex vs. placebo |
Week 24 Absolute change from baseline in BMI vs. placebo |
Week 24 Absolute change from baseline in Respiratory domain of CFQ-R vs. placebo |
Week 24 Absolute change from baseline in Sweat chloride vs. placebo |
|---|---|---|---|---|---|---|
| Elexacaftor- Tezacaftor- Ivacaftor |
One Phe508del- Minimal Function |
14.3 higher P < 0.001 |
63% lower P < 0.001 |
NA | 20.2 points higher P <0.001 |
41.8 mmol/L lower P < 0.001 |
| Week 4 Absolute change in ppFEV1 in ELX/TEZ/IVA vs. TEZ/IVA |
Week 4 Absolute change from baseline in Respiratory domain of CFQ-R in ELX/TEZ/IVA vs. TEZ/IVA |
Week 4 Absolute change from baseline in Sweat chloride in ELX/TEZ/IVA vs. TEZ/IVA |
||||
| Elexacaftor- Tezacaftor- Ivacaftor |
Homozygous Phe508del |
10.0 higher P < 0.0001 |
17.4 points higher P < 0.0001 |
45.1 mmol/L lower P < 0.0001 |
Unanswered Questions
Patients with Class I mutation who make up 10% of CF patients globally have yet to benefit from the existing modulator therapies. Class I mutations are nonsense mutations that cause in-frame premature termination codons (PTCs) that interruptribosomal translation and produce a truncated and nonfunctional CFTR protein. This activates a regulatory mechanism called nonsense-mediated mRNA decay that degrades PTC-containing mRNA, further decreasing the production of the shortened protein. Consequently, patients with Class I mutations have severe clinical phenotype (64).Ataluren, also known as PTC-124, is an oral compound that was tested for its ability to read through the mRNA resulting in full CFTR expression (64). Early results were encouraging, yet phase III trials concluded that Ataluren improved neither lung function nor exacerbation rate [62].
Pulmonary Outcomes
The existing CFTR modulators improve quality of life, lung function, and decrease morbidity in people with CF, who will invariably live longer. Nevertheless, questions remain as to the impact of partially restoring CFTR function on microbiology, inflammation, and the effects of non-pulmonary organs. Further uncertainties include the effect of CFTR modulators in the pediatric CF population, which is generally healthier and with little or no end-organ damage.For example: Would initiation of CFTR modulator therapies in CF during the first year of life delay or even prevent the onset of irreversible lung damage.If modulator therapies are started inolder children, with established yet mild disease, will they be at a lesser risk of developing chronic infections? Will the partial restoration of CFTR function obviate the need for other CF therapies? The bulk of the available data is on IVAsince it has been in use since 2012, and most of the studies are in adultsand adolescentssince approval for children came later.
Hisert and colleagues [63].studied 12 subjects before and following IVA therapy.The median age was 29.5 years, with a mean FEV1 of 64.2 %predicted.All the patients were cared for by the same CF center in Ireland.The decline in sputum P.aeruginosa began within 48 hours of IVA therapy and continued in the first year of treatment. However, no subject eradicated their infecting P. aeruginosa strain, and after the first year, P. aeruginosa densities rebounded.
The authors noted that sputum total bacterial concentrations also decreased, but less than P. aeruginosa. Sputum inflammatory measures diminished within the first week and continued to decline over two years. Chest CTs obtained before and one year of therapy demonstrated that IVA decreased airway mucous plugging. Rowe and co-workers [64].evaluatedCF patients six years of age and older who had positive cultures for P. aeruginosa 12 months before and 12 months after starting IVA. The proportion of subjects with P. aeruginosa significantly decreased from 55% (69 of 126) to 35% (38 of 108) in the 12 months on IVA.
Similarly, another study [65].demonstrated that IVAtherapy decreased Pseudomonas [mucoid and non-mucoid strains] and aspergillus and increased H. influenzae. These findings suggest a reversion to an earlier phenotype of CF microbiology as the mucoid phenotype is associated with chronic infections, and H. influenzae is more common in children than adults. A cohort study using longitudinalIrish CF Registry data concluded that IVA improved clinical outcomes while decreasing healthcare resource utilization (66).
On the other hand, Harris et al.[67].Evaluated 31 CF patients with a mean age of 27 years, and FEV1 > 41 % predicted. In this multicenter study, six months of IVA treatment were not associated with changes in airway microbial communities or indices of inflammation. These results suggest that despite effective CFTR modulator therapy, concomitant antimicrobial and anti-inflammatory treatments will be required to manage airway disease, especially in older patients with more advanced disease. The differences between this and other studies may have to do with differences in the CF populations, sputum processing, as well as baseline pseudomonas infection.
Non-Pulmonary Effects
Analyses of long‐term outcomes from registries around the globe offer insight into the non-pulmonary effects of IVA.Reductions in CF‐related diabetes [CFRD] and improved measures of bone health were noted in the US CF Foundation registry and the UK CF Registry, but not in the French CF Registry [68]. Kelly and colleagues studied 12 subjects, all with at least one gating mutation, all but one under 18 years of age. None of these participants had CFRD, exhibiting no or only mild glucose intolerance. The authors discovered that multiple measures of insulin secretion improved following four months of IVA treatment.While this was a small observational study, it alludes to the possibility of delaying and perhaps preventing the development of CFRD with early IVA therapy in some patients with CF[69].Hayes et al. [70],chronicled a 25-year-old pancreatic insufficient CF patient [deltaF508/G551D genotype] with a six years history of CFRDon insulin therapy. Following 13 months of IVA, the patient became normoglycemic with normal hemoglobin A1c levels and no longer required insulin.These data provide hope that restoration of CFTR function might treat and prevent CFRD. However, given the complexity of CFRD and the limited understanding of its pathogenesis, considerable uncertainty remains [71].
Gastrointestinal reports include a 17-year-old girl with CF [deltaF508/G551D] whose hepatic steatosis improved, at least by CT imaging, following two years of IVA therapy.The authors postulated that improvement was due to enhanced fat metabolism resulting in less hepatic fat accumulation (70). Gelfond and colleagues (72)assessed gastrointestinal pH and intestinal transit profiles in 10 CF patients with the G551D mutation. Following on month of IVA, patients demonstrated an increase in mean proximal intestinal pH, albeit there was no change in whole gut transit time. The authors concluded that improved bicarbonate secretion went along with CFTR restoration.These data further substantiate that CFTR is an essential regulator of bicarbonate as well as chloride.
Carrion et al. [73].carried out a retrospective observational study on six CF patients with a history of recurrent pancreatitis who were started on IVA therapy.All patients had one copy of deltaF508 and one copy of a mutation with residual CF function [Class III or IV]. The authors documented an impressive decrease in the frequency of pancreatitis.Indeed, none of the patients suffered an episode of pancreatitis following one year of IVA therapy. Patients required fewer opioids and fewer hospitalizations.
The data on the newer deltaF508 CFTR modulators are scant. In a retrospective analysis from a single CF center, Singh et al. demonstrated that CF patients receiving LUM/IVAhad significantly delayed acquisition of P. aeruginosa and S. aureus [74]. Another study concluded that LUM restored the ability of monocyte-derived macrophages from CF to phagocytose and kill P. aeruginosa to levels observed in monocyte-derived macrophages obtained from non-CF donors [75]. Kopp and co-workers conducted comprehensive blood RNA-seqtranscriptome analyses on 20 patients with CF [homozygous deltaF508, mean age 21.6 years, FEV1 74 %predicted] on 6-month LUM/IVA comparing them to 20 healthy controls. Their analysis revealed only modest changes in gene expression following LUM/IVA treatment[76].
The changes in microbiology and inflammation using the more effective triple combination in those with the delta F508 mutationhave yet to be studied.Nevertheless, thosewith irreversible organ damageare unlikely to stop non-modulator therapies.A White Paper by the CFFoundation suggests that patients with established lung disease will require daily therapy, including chest physiotherapy, mucolytics, and inhaled antibiotics. However, there will likely be much variability among individuals[77].
Cost
IVA costs approximately £180,000 or US$234280 annually.This staggering cost may well impact the financial stability of health systems since modulators will be started at a young age and taken for the life of the individual(2).Economists analyze cost-effectiveness by calculating quality-adjusted life-year [QALY] gained, and QALY gain for IVA cross the benchmark set by the UKNational Health Service. The arguments about what constitutes value and how do CF providers, insurers, and governments incorporate equity, cost, and value into decision making are complex (2).
Conclusions
In this review, we outlined the progress in CF therapyfrom nebulized recombinant human DNAse, approved in 1994, to the triple oral combination of ELX/TEZ/IVA approved in 2019. Initial therapies along with standardized care in accredited CF centers, the creation of CF registries to track the disease and a commitment to quality improvement, changed CF from a fatal disease of children to a chronic, albeit still fatal, illness of young adults.The introduction of oral agents with acceptable side effects that target the protein defect may well render CF a non-fatal disease. Indeed,CF has become a model for other chronic, rare diseases.Challenges remain, including modulator therapy for Class I and other rare mutations as well as cost and access to care.
References
1. Stevens DP, Marshall BC. A decade of healthcare improvement in cystic fibrosis: Lessons for other chronic diseases. BMJ QualSaf. 2014;23(SUPPL1):2–4.
2. Cystic Fibrosis Foundation. Patient Registry Annual Data Report.Annual Data Report.Bethesda, MD; 2018. Available from: http://www.cff.org/UploadedFiles/research/ClinicalResearch/Patient-Registry-Report-2009.pdf
3. Corriveau S, Sykes J, Stephenson AL. Cystic fibrosis survival: the changing epidemiology. CurrOpinPulm Med. 2018;24(6):574–8.
4. Riordan JR, Rommens JM, Kerem BS, et al.Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–73.
5. Boyle MP, De Boeck K. A new era in the treatment of cystic fibrosis: Correction of the underlying CFTR defect. Lancet Respir Med. 2013;1(2):158–63.
6. De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med. 2016;4(8):662–74.
7. Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis.BMJ. 2016;352:1–14.
8. Holguin F. Triple CFTR modulator therapy for cystic fibrosis. N Engl J Med. 2018;379(17):1671–2.
9. Elborn JS. Cystic fibrosis.Lancet. 2016;388:2519–31.
10. Felix Ratjen, Scott C. Bell, Steven M. Rowe, et al. Cystic Fibrosis. Nat Rev. 2015;1(May):1–19.
11. McIlwaine MP, Alarie N, Davidson GF, et al. Long-term multicentrerandomised controlled study of high frequency chest wall oscillation versus positive expiratory pressure mask in cystic fibrosis. Thorax. 2013;68(8):746–51.
12. Feldman M, Cryer B. Effects of Aeroslolized Recombinant Human DNase on Exacerbations of Respiratory Symptoms and on Pulmonary Function in Patients with Cystic Fibrosi. N Engl J Med. 1994;331(10):637–42.
13. Elkins MR, Sc MH, Robinson M, Ph D, et al.A Controlled Trial of Long-Term Inhaled Hypertonic Saline in Patients with Cystic Fibrosis. N Engl J Med. 2006;354(3):229–40.
14. Burness CB, Keating GM. Mannitol Dry Powder for Inhalation. Drugs. 2012;72(10):1411–21.
15. Flume PA, Aitken ML, Bilton D, et al. Optimising inhaled mannitol for cystic fibrosis in an adult population. Breathe. 2015;11(1):39–48.
16. Fiel SB. Aerosolized antibiotics in cystic fibrosis: an update. Expert Rev Respir Med. 2014;8(3):305–14.
17. Ramsey BW, Pepe MS, Quan JM, et al. Intermittent Administration Of Inhaled Tobramycin In Patients With Cystic Fibrosis. N Engl J Med. 1999;340:23–30.
18. McCoy KS, Quittner AL, Oermann CM, et al. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J RespirCrit Care Med. 2008;178(9):921–8.
19. Retsch-Bogart GZ, Quittner AL, Gibson RL, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest. 2009;135(5):1223–32.
20. Konstan MW, Flume PA, Kappler M, et al. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J Cyst Fibros. 2011;10(1):54–61.
21. Flume PA, Clancy JP, Retsch-Bogart GZ, et al. Continuous alternating inhaled antibiotics for chronic pseudomonal infection in cystic fibrosis. J Cyst Fibros. 2016;15(6):809–15.
22. Griffith DE, Eagle G, Thomson R, Aksamit TR, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT) a prospective, open-label, randomized study. Am J RespirCrit Care Med. 2018;198(12):1559–69.
23. Clancy JP, Dupont L, Konstan MW, et al. Phase II studies of nebulisedArikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68(9):818–25.
24. Bilton D, Pressler T, Fajac I, et al. Amikacin liposome inhalation suspension for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J Cyst Fibros. 2019;000:6–11.
25. Khoury O, Barrios C, Ortega V, et al. Immunomodulatory Cell Therapy to Target Cystic Fibrosis Inflammation. Am J Respir Cell Mol Biol. 2018;58(1):12–20.
26. Nichols DP, Durmowicz AG, Field A, et al. Developing inhaled antibiotics in cystic fibrosis: Current challenges and opportunities. Ann Am Thorac Soc. 2019;16(5):534–9.
27. Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev. 2017;2017(4).
28. Saiman L. Improving outcomes of infections in cystic fibrosis in the era of CFTR modulator therapy.PediatrPulmonol. 2019;54:S18–26.
29. Cramer CL, Patterson A, Alchakaki A, et al. Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad Med. 2017;129(5):493–9.
30. Wolter J, Seeney S, Bell S, et al. Effect of long-term treatment with azithromycin on disease parameters in cystic fibrosis. Thorax. 2002;57:212-216.
31. Saiman L, Marshall BC, Mayer-hamblett N, et al. Azithromycin in Patients With Cystic Fibrosis Chronically Infected with Pseudomonas aeruginosa. J Am Med Assoc. 2003;290(13):1749–56.
32. Ratjen F, Saiman L, Mayer-Hamblett N, et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest. 2012;142(5):1259–66.
33. Nichols DP, Happoldt CL, Bratcher PE, et al. Impact of azithromycin on the clinical and antimicrobial effectiveness of tobramycin in the treatment of cystic fibrosis. J Cyst Fibros. 2017;16:358–66.
34. Nick JA, Moskowitz SM, Chmiel JF, et al. Azithromycin may antagonize inhaled tobramycin when targeting Pseudomonas aeruginosa in cystic fibrosis. Ann Am Thorac Soc. 2014;11(3):342–50.
35. ayer-Hamblett N, Retsch-Bogart G, Kloster M, et al. Azithromycin for early pseudomonas infection in cystic fibrosis the OPTIMIZE randomized trial. Am J RespirCrit Care Med. 2018;198(9):1177–87.
36. Trend S, Fonceca AM, Ditcham WG, et al. The potential of phage therapy in cystic fibrosis: Essential human-bacterial-phage interactions and delivery considerations for use in Pseudomonas aeruginosa-infected airways. J Cyst Fibros. 2017;16(6):663–70.
37. Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25(5):730–3.
38. Goss CH, Kaneko Y, Khuu L, et al. Gallium disrupts bacterial ion metabolism and has therapeutic effects in mice and humans with lung infections. SciTransl Med. 2018;10(460):1–11.
39. De Groote MA, Fang FC. NO inhibitions: Antimicrobial properties of nitric oxide. Clin Infect Dis. 1995;21:17–20.
40. Howlin RP, Cathie K, Hall-Stoodley L, et al. Low-Dose Nitric Oxide as Targeted Anti-biofilm Adjunctive Therapy to Treat Chronic Pseudomonas aeruginosa Infection in Cystic Fibrosis. MolTher. 2017;25(9):2104–16.
41. Roesch EA, Nichols DP, Chmiel JF. Inflammation in cystic fibrosis: An update. PediatrPulmonol. 2018;53(April):S30–50.
42. Konstan MW. Therapies aimed at airway inflammation in cystic fibrosis. Clin Chest Med. 1998;19(3):505–13.
43. Balfour-Lynn IM, Welch K, Smith S. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev. 2012;(11).
44. Konstan MW, VanDevanter DR, Sawicki GS, et al. Association of high-dose ibuprofen use, lung function decline, and long-term survival in children with cystic fibrosis. Ann Am Thorac Soc. 2018;15(4):485–93.
45. Lands LC, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2019;2019(9).
46. A Trial to Evaluate Efficacy and Safety of Lenabasum in Cystic Fibrosis. [cited 2019 Nov 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT03451045
47. Elborn JS, Ahuja S, Springman E, et al. EMPIRE-CF: A phase II randomized placebo-controlled trial of once-daily, oral acebilustat in adult patients with cystic fibrosis – Study design and patient demographics. ContempClin Trials. 2018;72(April):86–94.
48. Pranke I, Golec A, Hinzpeter A, et al. Emerging therapeutic approaches for cystic fibrosis. From gene editing to personalized medicine. Front Pharmacol. 2019;10(FEB):1–21.
49. Ramsey BW, Davies J, McElvaneyG , et al. TV-770-102 SG. A CFTR Potentiator in Patients with Cystic Fibrosis and the G551D Mutation. N Engl J Med. 2011;365(18):1663–72.
50. Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor–ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med. 2017;377(21):2024–35.
51. Van Goor F, Yu H, Burton B, et al. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 2014;13(1):29–36.
52. Guigui S, Wang J, Cohen RI. The use of ivacaftor in CFTR mutations resulting in residual functioning protein.Respir Med Case Reports. 2016;19.
53. FDA expands approved use kalydeco to treat additional mutations in cystic fibrosis.. Available from: https://www.fda.gov/news-events/press-announcements/. [Cited 2019 Dec 20]
54. Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. N Engl J Med. 2015;373(3):220–31.
55. Elborn JS, Ramsey BW, Boyle MP, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. Lancet Respir Med. 2016;4(8):617–26.
56. Konstan MW, McKone EF, Moss RB, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5(2):107–18.
57. Talamo Guevara M, McColley SA. The safety of lumacaftor and ivacaftor for the treatment of cystic fibrosis.Expert Opinion on Drug Safety. 2017;16: 1305–11.
58. Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377(21):2013–23.
59. Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–19.
60. Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–8.
61. ZainalAbidin N, Haq IJ, Gardner AI, et al. Ataluren in cystic fibrosis: development, clinical studies and where are we now? Expert OpinPharmacother. 2017;18(13):1363–71.
62. Hisert KB, Heltshe SL, Pope C, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J RespirCrit Care Med. 2017;195(12):1617–28.
63. Rowe SM, Heltshe SL, Gonska Tet al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiatorivacaftor in G551D‐mediated cystic fibrosis. 2014;190(2):175-184. Am J RespirCrit Care Med. 2014;190(2):175–84.
64. Heltshe SL, Mayer-Hamblett N, Burns JL, et al. Pseudomonas aeruginosa in Cystic Fibrosis Patients With G551D-CFTR Treated With Ivacaftor. Clin Infect Dis. 2015;60(5):703–12.
65. Kirwan L, Fletcher G, Harrington M, et al. Longitudinal trends in real-world outcomes after initiation of ivacaftor: A cohort study from the cystic fibrosis registry of Ireland. Ann Am Thorac Soc. 2019;16(2):209–16.
66. Harris JK, Wagner BD, Zemanick ET, et al. Changes in Airway Microbiome and Inflammation with Ivacaftor Treatment in Patients with Cystic Fibrosis and the G551D Mutation. Ann Am Thorac Soc. 2019;1–47.
67. Savant AP, McColley SA. Cystic fibrosis year in review 2018, part 1. PediatrPulmonol. 2019;54(8):1117–28.
68. Kelly A, De Leon DD, Sheikh S, et al. Islet hormone and incretin secretion in cystic fibrosis after four months of ivacaftor therapy. Am J RespirCrit Care Med. 2019;199:342–51.
69. Hayes D, Warren PS, McCoy KS, et al. Improvement of hepatic steatosis in cystic fibrosis with ivacaftor therapy. J PediatrGastroenterolNutr. 2015;60(5):578–9.
70. Norris W. Is Cystic Fibrosis–related Diabetes Reversible? New Data on CFTR Potentiation and Insulin Secretion. Am J RespirCrit Care Med. 2019;199(3):261–253.
71. Gelfond D, Heltshe S, Ma C, et al. Impact of CFTR modulation on intestinal pH, motility, and clinical outcomes in patients with cystic fibrosis and the G551D mutation. ClinTranslGastroenterol. 2017;8(3):e81-6.
72. Carrion A, Borowitz DS, Freedman SD, et al. Reduction of Recurrence Risk of Pancreatitis in Cystic Fibrosis with Ivacaftor: Case Series. J PediatrGastroenterolNutr. 2018;66(3):451–4.
73. Singh SB, McLearn-Montz AJ, Milavetz F, et al. Pathogen acquisition in patients with cystic fibrosis receiving ivacaftor or lumacaftor/ivacaftor. PediatrPulmonol. 2019;54(8):1200–8.
74. Barnaby R, Koeppen K, Nymon A, et al. Lumacaftor (VX-809) restores the ability of CF macrophages to phagocytose and kill Pseudomonas aeruginosa. Am J Physiol - Lung Cell Mol Physiol. 2018;314(3):L432–8.
75. Kopp BT, Fitch J, Jaramillo L, et al. Whole-blood transcriptomic responses to lumacaftor/ivacaftor therapy in cystic fibrosis. J Cyst Fibros.Available online Aug 2019.
76. Cystic Fibrosis Foundation. CFTR modulators for the treatment of cystic fibrosis.Whitepaper. 2019.
Received: December 07, 2019;
Accepted: January 13, 2020;
Published: January 15, 2020.
To cite this article : Rubin.C.Cystic Fibrosis Therapy: From Chest Physiotherapy to Agents Targeting Specific Mutations. European Journal of Respiratory Medicine. 2020: 1:2.
©Rubin.C. 2020.
