Research Article / Open Access
DOI: 2020; 2(1): 152 - 162. doi: 10.31488/ejrm.110
Evidence-Based Practice for Care of Preterm Infants Receiving Non-Invasive Ventilation (Clinical Trials identifier: NCT04165382)
Chan SY*1, Professor Janita PC Chau2
1. Student of Doctor in Nursing, The Nethersole School of Nursing, The Chinese University of Hong Kong (CUHK), Hong Kong
2. The Nethersole School of Nursing, Faculty of Medicine, CUHK, Hong Kong
*Corresponding author: Chan SY, Student of Doctor in Nursing, The Nethersole School of Nursing, The Chinese University of Hong Kong (CUHK), Hong Kong
Abstract
Background: The primary strategy for mechanical ventilation in NICU has been evolved in recent twenty years, the non-invasive ventilation (NIV) support become the mainstay for preterm infants. It prevents different ventilator induced lungs injury, however, complications such as nasal necrosis, nasal disfiguration and abdominal distension were reported in several studies. Nevertheless, many studies compared the efficacy of invasive ventilation and NIV on treatment of different respiratory problems found in the preterm infants. Few studies explored the practice on care of these infants receiving NIV. This study aims to search relevant empirical findings and developed an evidence-based clinical practice guideline for the preterm infants receiving NIV. Method: The Iowa Model was adopted as the theoretical framework to guide the guideline development process. A workgroup consists of multidisciplinary healthcare providers is formed for the purpose. Literature search from eight electronic databases are performed, and the John Hopkins University’s evidence appraisal tool was used to assess the quality of the evidence.Result: 16 eligible articles are identified: six systematic reviews, seven randomized clinical trials, one quasi-experimental study and two cohort studies. In addition, two clinical guidelines from overseas hospitals are also found. The recommended practices include six core elements: 1) right choice of nasal interfaces (evidence level 1B,C, IIB, IIIB); 2) regular alternating the nasal interfaces (evidence level IB,C & IIIA, B); 3) use of skin protective dressing on the nasal pressure areas (evidence level IIIA,B,C); 4) regular positioning the infant (evidence level IIC, IIIB); 5) frequent skin assessment (evidence level IC, IIIB); 6) supportive cares including lubricate the prongs with saline before putting into the nostrils (evidence level IIIB); gently massage the pressure areas without any ointment (evidence level IIIB); avoid unnecessary nasal suction (evidence level IIIB), and provide adequate humidification to the ventilator circuit (evidence level IIIB).Conclusion: The clinical practice guideline will be implemented in a local NICU of 23 level III care beds. A before and after study will be conducted, and it aims to examine the effects of the guidelines on infant’s pain level, time in sleeping or quiet state, and incidence of nasal injury during receiving NIV.
Background
Most of infants admitted to the neonatal intensive care unit (NICU) are preterm infants who born before the completion of gestation age at 37 weeks. These infants usually require different respiratory supports due to their immature lungs’ development. The respiratory supports include supplemental oxygen and mechanical ventilation. In the past, the invasive ventilation was the primary strategy for the mechanical ventilation to the infants who have severe respiratory problems, especially the preterm infants in NICU. The preterm infants born with immature lungs structure, were unable to product sufficient surfactant to expand all lung alveoli for gaseous exchange. It led to low oxygenation that required immediate intubation for surfactant replacement therapy. After the therapy, they were usually continued the invasive ventilation with endotracheal tube in place. However, it was likely to result in complications of ventilator induced lungs (VILI) injury such as barotrauma, volutrauma, and bronchopulmonary dysplasia (BPD) [1,2]. Subsequently, it prolonged the time of ventilation and intubation to the infants and caused burden to both healthcare sector and the family.
In view of the impact of adverse effects on infants receiving invasive ventilation, the early NIV in form of continuous positive airway pressure (CPAP) support was introduced to NICU by Gregory in 1971 [1,3,4], it aimed to provide an alternative ventilation support to the infants and minimize VILI at simultaneously. With advancing of the ventilator technologies, more support modes in NIV possess similar pressure support functions as in invasive ventilation including Intermittent Positive Pressure Ventilation (IPPV) and Neurally Adjusted Ventilatory Assist (NAVA); Hence, NIV becomes the mainstay of ventilation strategy in NIV, and European Consensus Guidelines on the management of respiratory distress syndrome and American Academy of Pediatrics also recommended to use NIV to the preterm infants who required respiratory supports at birth [5,6]. Moreover, many studies had explored the efficacy of NIV and compared its adverse outcomes such as BPD and mortality with invasive ventilation. Empirical findings showed that the failure of NIV in treating different infant’s respiratory problems were not significantly difference from invasive ventilation, and the mortality and incidence of BPD in infants receiving NIV was significantly less than invasive ventilation [7-12]. Nevertheless, many studies reported complications such as nasal injury [13-16], forehead lesion [17] and abdominal distension [2,18,19] in infants receiving NIV. Studies for evidence-based nursing interventions on preterm infants receiving NIV were few, and related nursing interventions were mentioned in many studies but findings only revealed severity of nasal injury associated from NIV under a bundle of care, and further analysis on the effects of each intervention to the complications were scarcity.
This review aims to identify empirical studies which included relevant nursing interventions to care of preterm infants receiving NIV, and then to compile the found interventions to develop a clinical practice guideline for further implementation and evaluation.
Method
The Iowa Model was used as the theoretical framework to guide the guideline development. This model is chosen as it is well-adopted in different clinical areas in development of evidence-based practices, it allows revise and modify any step along the process at different time points whenever it is necessary [20]. It is feasible and practical for clinical situation which is agility and subjected to change according to ad hoc situation.
Prior to finding the evidence, a workgroup of multidisciplinary healthcare professional is established for the purpose. There are eight members involving one nurse consultant in neonatal care; three advanced practice nurses who are experts in NICU for ventilator care, developmental care and wound care; one ward manager, one registered nurse with the specialty experiences over 20 years; one consultant of neonatology, and one senior physiotherapist working in NICU. They provide expert opinion in the process and promote the new guideline to NICU by acting as a facilitator for its implementation in daily operation.
Afterwards, literature search was conducted from eight electronic databases including Cochrane Library, EMBASE, Medline, PubMed, CINAHL, OVID, Joanna Briggs Institute EBP database via OVIDSP, Maternity and Infant Care Database and Grey Literature Report. In addition, the websites of seven institutions were navigated to find out relevant information to supplement the reviews, the institutions include National Guideline Clearing House, British Thoracic Society, American Academy of Paediatrics, Society of Obstetricians and Gynaecologists of Canada, Royal Australian and New Zealand College of Obstetrician and Gynaecologists, Royal College of Obstetrician and Gynaecologists, and The Royal Children’s Hospital Melbourne (RCH) [52].
The “PICOs” search strategy was used in the process, and terms matching to subject heading and keyword included: Patient – “infant, low birth weight infant, premature infant, extremely premature infant, very low birth weight infant, extremely low birth weight, premature birth or baby or newborn or neonate, preterm infant or baby or neonate or newborn; respiratory distress syndrome, newborn irds or rds, hyaline membrane disease or hmd, transient tachypnea of the newborn, respiratory distress, apnoea of prematurity”. Intervention & Comparison – “non invasive ventilation, positive-pressure respiration, artificial respiration, continuous positive airway pressure, intermittent positive-pressure ventilation, nasal respiration ventilation, nasal continuous positive airway pressure, npcpap, nasal intermittent positive pressure ventilation, nippv, bipap; nasal interface, nasal device, nasal prong, binasal prong, nasal cannula, nasal mask; hospital teaching, in service training or teaching. staff development or education, nursing education, professional education, continuing education; guideline, nurse guideline, clinical practice guideline, nursing care, nursing management, nursing strategy, nursing practice, evidence-based practice or protocol”. Outcome - “skin ulcer or breakdown, pressure ulcer or injury, device related pressure injury, soft tissue injuries or wounds, nasal injury, nasal septal injury, nasal sore or necrosis, nasal skin breakdown, nose injury or sore or necrosis or deformities”. study – “clinical experimental study, randomised controlled trial (RCT), cohort study, quasi-experimental study, systematic review, integrated review”.
After identified eligible studies from the search, The John Hopkins University’s evidence appraisal tool (2017) was used to assess the quality of the evidence. This appraisal tool classifies evidence into five levels as: Level I - experimental study, randomized controlled trial, explanatory mixed method design and systematic review of RCTs; Level II - quasi-experimental study, explanatory mixed method design, and systematic review of a combination of RCTs and quasi-experimental studies, or quasi-experimental studies only; Level III - nonexperimental study, systematic review of a combination of RCTs, quasi-experimental and non-experimental studies, or nonexperimental studies only, exploratory, convergent, or multiphasic mixed methods studies, explanatory mixed method design, qualitative study meta-synthesis; Level IV - opinion of respected authorities and/or nationally recognized expert committees or consensus panels based on scientific evidence; Level V - experiential and non-research evidence [21].
For the evidence quality, it rates the quality of quantitative studies at three grades as A: high quality which indicates consistent, generalizable results from the study design of sufficient sample size and adequate control, with definitive conclusions and consistent recommendations; B: good quality which indicates reasonable consistent results from the study design of sufficient sample size and some control, with fairly definitive conclusions and reasonable consistent recommendations; C: low quality or major flaws which indicates little evidence with inconsistent results from the study design of insufficient sample size, without conclusions drawn [21].
Results
There were totally 143 studies identified from the databases, and five studies were added after scrutinised the references of the identified studies. After scrutinised title and abstract of the identified studies as well as reviewed the study design, 132 studies were excluded due to duplication (74 studies), irrelevant (42 studies), Level V evidence (15 studies), and printed in Korean (1 study). Finally, there are 16 eligible studies for quantitative synthesis (Figure 1). It includes six systematic reviews including (evidence Level II to III); seven RCTs (evidence Level I), 1 quasi-experimental study (evidence level II), and two cohort studies (evidence level III) (Supplementary Table 1). For the evidence quality among the six systematic reviews, there is one grade A whereas others are all grade B. For the seven RCTs, there are four evidence at grade B whereas the remained three are at grade C. The low grading for the studies were mainly because of: not clearly mentioned of sample size calculation in the methodology; numbers of subject for the groups below the target numbers; or underpowered of the sample size to detect the difference between study groups so that it might be insufficient to generalise the results. For the quasi-experimental study, its evidence quality is grade C as the sample size calculation was also not stated in the methodology, and it might be insufficient for generalisation of the results. The evidence quality for the two cohort studies is at grade B.
In addition, two clinical practice guidelines (Level IV evidence) were found from the seven institutions websites, and these two guidelines were included in the evidence appraisal. The quality of these two guidelines is at grade C as both did not clearly stated the appraisal of the evidence where the guideline was based on for the establishment.
Although there are some evidence of grade C quality, at the point of good practice for the clinical area, those evidence are included. The interventions identified from the 16 studies are mainly summarised as six core elements: choice of nasal interfaces; use of skin protective dressing; regular alternating the interfaces; regular positioning the infant; frequent of skin assessment; and other supportive cares.
Choice of nasal interfaces
One study [22] found that use of short binasal prongs was significant lower treatment failure than other interfaces (RR0.63, 95% CI: 0.4-097) (level IIB). Moreover, three studies [23-25] found that nasal mask was significantly less severity in nasal injuries than the prongs (p=0.01-0.05) (level IB, IIB, IC). On the other hand, three studies [26-28] did not find significant difference in nasal injury between the prongs & mask (p=0.0946-0.5) (level IB). In addition, two systematic reviews [16, 29] identified that incorrect sizing and positioning of the nasal interface were the common risk factors for the nasal injury (level IIIB).
Use of skin protective dressing
Two studies [26,28] and one systematic review [29] identified that the common nasal areas for device-related pressure injured included the junction between the base of nasal septum and the philtrum for nasal mask whereas the medial aspect of nostrils and the columella for nasal prongs (level IB & IIIB).
One study [30] and four systematic reviews [16,29,31,32] revealed that there was significant difference in reducing nasal injuries when skin protective layers/dressing such as silicone gel sheet (p<0.05) (level IIIA, IIIB) or hydrocolloid dressing (p=0.01) (level IIIB & IC) was used for the nasal areas; or prophylactic dressing underneath the interfaces (p<0.05) (level IIIB). Moreover, one study [15] also shown that use of skin protective layer resulted in less severity of nasal trauma (88.3% stage I trauma, 11% stage II, 0.7% stage III trauma) (level IIIB).
Regular alternating nasal interfaces: short binasal prongs with mask
One study [33] and 1 systematic review [31] identified that less frequent and severe of nasal erythema (p<0.001) and excoriation (p=0.007) were found in the group of rotation application of nasal interfaces for NIV (level IIIA & IIIC). It was further reinforced by one study [15] and one systematic review [16] that less severity of nasal injury was found for alternating the nasal prongs with mask every 4-6 hours, there were about 29% without trauma; 62% to 88.3% at stage I trauma, 9% to 11% stage II, and 0.7% stage III trauma (level IIIB).
On the other hand, one study [26] found that an equal occurrence of nasal trauma in prongs and mask (p=0.0946) (level IB)
Regular positioning the infant
One study [34] found that limited number in changing of position was significant risk factor for nasal injuries in infants receiving the NIV (p<0.05) (level IIIB). Moreover, one study [35] revealed that displacement of nasal prong was higher occurrence in prone position (56.2%, P=0.001) than in left lateral position (12.5%) or supine (0%) (level IIC). Furthermore, another study [15] also identified that positioning infants in supine or on sides had less severity of nasal injury (88.3% of stage I trauma, 11% of stage II, 0.7% of stage III trauma) (level IIIB).
Frequent skin assessment
One study [15] found that less severity of nasal trauma was noted for infants when skin assessment was performed every 30 to 60 minutes and the device was removed to close inspect underneath skin areas every two to four hours (88.3% stage I trauma, 11% stage II, 0.7% stage III trauma) (level IIIB). Moreover, one study [33] also found that frequent and severe of erythema (p<0.001) and excoriation (p=0.007) were more less observed when initial nasal skin assessment on infant was performed within 8 hours after extubation to the NIV, then performed every 10-12 hours (level IC). Furthermore, two systematic reviews [29, 32] recommended to assess skin areas under and around medical device regularly at least twice daily for minimizing the occurrence of nasal trauma (level IIIB).
For the skin assessment tools used in the studies, no standard instrument was used among the studies. There was one study [15] that used a modified scale developed from the US National Pressure Ulcer Advisory Panel (NPUAP) to measure the severity of nasal trauma. The trauma was classified at three stages: stage I – persistent erythema; stage II – superficial ulceration; and stage III – necrosis (level IIIB). Besides, another study [33] used the National Skin Condition Scale (NSCS) to rate the skin condition in three aspects as dryness, erythema, breakdown/ excoriation. Scoring in each aspect was from one to three that one indicated healthy skin condition whereas two to three indicated increase of severe skin breakdown. The total score was minimum at three and maximum at nine (level IC).
Other supportive cares
Fischer, et al. [15] found that gentle massage with ointment over common pressure points over the nasal areas every 2-4 hours had resulted in less severity of nasal trauma to the infants (88.3% stage I trauma, 11% stage II, 0.7% stage III trauma) (level IIIB). Haesler [32] also recommended to select right size of NIV interface and device as well as regularly moisturize the skin underneath the device to reduce the risks of nasal injury (level IIIB). In addition, a systematic review [29] suggested to avoid unnecessary suctioning and provide adequate humidification to the ventilator circuit to prevent nasal injury (level IIIB).
Other than the findings of the empirical studies, the two nursing guidelines also added some interventions for care of infants receiving NIV, it included proper securing device not to cause any indentation, pitting or periorbital oedema on the infant; to empty accumulated condensation in the ventilator tubing to minimize the risks for nasal injury; to regularly decompress the stomach gas by insertion of an orogastric tube every four to six hour to relieve the abdominal distension; or free drainage the tube half hour after feed (a practice mentioned in the study of Goel, et al., 2015); to use a pacifier or chin strap to close the mouth to achieve optimal pressure of NIV (level IVC).
Discussion
With the six core findings summarised from the 16 empirical studies, the nursing practice are developed with the aims to sustain optimal NIV pressure; to minimise pressure injury arisen from the device; and to promote infant’s comfort for receiving NIV. Firstly, for the core element “choice of nasal interface”, it shown that choice of a short binasal prongs was better to reduce the failure of NIV than other prongs. However, there was no consensus among the studies for using nasal prongs or mask in infants receiving NIV. McCoskey, et al. [36] and Flanagan [37] also stated that no one nasal interface (nasal prongs or nasal mask) was superior to other. Despite the choice of nasal interface, it is important to choose a right size of interface to fit the infant’s nose. As Nascimento et al. [38] and Badr, et al. [39] explained that smaller prongs did not fit the nostrils, and moving of the loosen prong-ends insides the nostrils caused more fiction to surrounding skin/ mucosa whereas larger prongs enlarged the nostrils, and distorted the nostrils to the prong size. In long term, it might result in either nasal trauma or deformity of the nasal structure. Besides, Sorensen, et al. [40] found that nurses usually chosen the nasal interface and anchoring bonnet based on their experiences instead of using actual measures on the size of nose and head circumference for fitting. Thus, the interface might not fit the infant that achieving the constant and optimal pressure was not possible by NIV [41]. Hence, no matter which nasal interface is used, to select a “right size of nasal interface” should be reinforced in the nursing practice. The nasal interface should be chosen based on the measures of nose size including the width of the columella, and the manufacturer’s recommendation. In addition, some studies [17, 38] found that non-fitted anchoring cap / bonnet was unable to stabilise the ventilator tubing on infant’s head, it caused traction to the forehead or the nose and resulted in pressure injury on the forehead, glabella or nostrils. Besides, undersized cap might induce fiction to the scalp and caused scalp necrosis. Therefore, right size of anchoring bonnet should also be selected according to the measures of infant’s head circumference.
Secondly, for use of skin protective dressing, all related findings in the 16 studies shown reduction of the incidence or severity of nasal injury after use of the dressing. Several other studies regarding the prevention of pressure injury also identified the benefits of using the skin protective dressing such as silicon sheet or hydrocolloid film [39, 42,43]. Moreover, as indicated in the findings, there were certain common pressure areas for different nasal interface used in NIV, so it needs to apply the skin protective dressing over the related areas accordingly.
Thirdly, for regular alternating the nasal interfaces for NIV, although manufacturers recently improved nasal interfaces by using softer materials and being more flexible [37], prolonged applying one interface for NIV would increase pressure on the contacted surfaces. It is necessary to remove the interface to rest the skin periodically, however, this practice is not possible in infant, especially at preterm infants. Therefore, rotation of using different nasal interface in the infants receiving NIV is more feasible to relieve pressure over the areas. Findings of the 16 studies also shown the effects of this practice on the severity of nasal injury. In addition, Kieran, et al. [26], Haesler [32] and Newnam, et al. [29] recommended in the study to alternate and reposition the nasal interfaces on a regular basis to alter the pressure points over the nose. Besides, Newnam, et al. [29] also emphasized to shape the prongs at best align with the physiological angle to the nostrils; and moisten the prongs with sterile saline before putting the prongs into infant’s nostrils.
Fourthly, for regular positioning the infant, the findings identified that positioning infants with NIV to prone was higher risks in occurrence and increase of severity in nasal injury due to its mostly displacement of the interface. Although, some studies stated that prone promoted better oxygenation and stomach emptying, it was not recommended due to the risk of sudden infant death syndrome or presence of umbilical catheters or abdominal surgical wound [37, 44, 45]. Moreover, regular positioning was necessary which was mentioned in Flanagan [45], and Baharrestani et al. [46] as it helped to prevent pressure injury, or asymmetrical, flattened, or misshapen head in infants. Furthermore, turning should be kept at developmental position that head to trunk was in a midline with upper limbs placing slightly closed to the body when infants were keeping at supine or lying aside, and this practice was also recommended by Newnam, et al. [29] in the 16 studies. In addition, some nasal interfaces were “midline” designed that the ventilator tubing was attached at the midline to the infant’s head instead of the sides of the head and faces. It enables lying the infant at any side other than the supine. [47], thus, use of the midline nasal interface was preferrable to facilitate regular positioning the infant.
Fifthly, for frequent skin assessment, NIV pressure is frequently varied and difficulty maintained in real clinical situation. The pressure fluctuation is mainly due to the massive leakage from the displaced interface or opening of infant’s mouth. In view of the causes, nurses tend to tightly tie the anchoring straps onto infant’s faces to keep the interface in position, it consequently causes excessive compression onto the contacted areas over the nose. Moreover, checking device position was found not frequently enough, then it further increases risks of injury associated with improper position of the device [48-50]. Therefore, one of the nursing practices concluded from the 16 studies: “frequent nasal skin assessment” can reduce nasal injury as this practice enables early detection of interface displacement and allows to relieve the compression over the pressure areas when the device is removed to inspect underneath skin. For the instruments used for skin assessment and staging of pressure injury, no standardised instrument was identified across the studies. To enable generalisation of the findings in these aspects for infants of NICU, reliable and valid instruments are recommended to use for the purpose.
Finally, for other supportive cares, interventions recommended in the studies were purposely used to alleviate some risk factors of nasal injury, it included fiction or shearing, moisture, and perfusion [46], and these interventions were similarly stated in the two clinical guidelines.
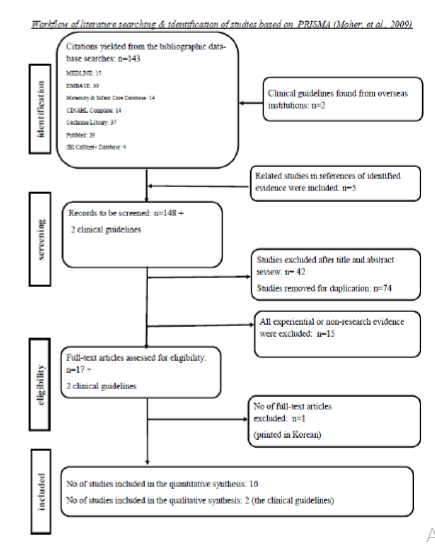
Figure 1.Workflow of literature searching & identification of studies based on PRISMA [36]
Table 1. Appraisal of the evidence
| Authors (Year) Evidence level & quality | Study design | sample size & setting | Interventions identified related nursing care/recommendation or for NIV |
Findings | Limitations |
|---|---|---|---|---|---|
| August, et al., (2018) IIIA | Systematic review | 21 studies of RCT & cohort sample size from 32 to 1033 infants |
• nasal prongs, mask or alternative rotation were used for NIV • silicone gel sheeting was applied to device contacted areas in one study |
• prevalence of neonatal skin injury ranged from 9.25 to 43.1% • risk factors included: o medical devices: > 70% o gestational age < 32 weeks: OR2.48, 95% CI 1.59-3.86, p<0.001 o weight:< 1500g: OR 2.28, 95% CI 1.43-3.64, p<0.001 • injuries related to respiratory interfaces: nasal areas: 20%-100% mask: 29% prong: 35% forehead: 26.6% |
N/A |
| N/A | Quasi- experimental study | 16 infants of mean GA at 29.7+/- 2 weeks, BW of 1353+/-280g,
days of life 2.9+/-2.2 days in a Paed. ICU sector of a tertiary public hospital in Brazil |
infants were put in 4 sequences of positioning & each positioning was kept for 60 minutes A: supine B: right later C: prone D: left lateral Sequence1: A,D,C,B Sequence 2: C,A,B,D Sequence 3: B,C,D,A Sequence 4: D,B,A,C • hydrocolloid in nostrils was applied to keep the orifices open for introduction of the nasal prongs • size of the prongs was chosen as per the reference table provided by the manufacturer according to child’s weight & diameter of the devices • the gases for NIV was humidified & warmed • cushion supports were provided for body positions at left & right lateral positions |
- displacement of nasal prong at prone position: 56.2% (p=0.01) left lateral position: 12.5% - increased requirement on device correction in the nostrils for the displacement at prone and the left lateral positions |
- details of the sample size calculation were not stated - sample size might be insufficient for generalisation of the results |
| Chandrasekaran, (2017) IB | RCT | 72 neonates of GA 26
to 32 weeks 3 teaching hospitals in India |
provided NCPAP via nasal mask or binasal prongs | incidence of severe nasal trauma was significant lower in the mask group (0%) than binasal prong group (31%), p=0.01 | no blinding in the study |
| De Paoli, et al., (2008) IIB | Cochran review | 7 studies of RCT & quasi-randomised trials 589 infants at GA less than 36 weeks, of BW less than 2500g in different hospital setting |
provided NCPAP via different nasal interface nasal mask short single prong either nasopharyngeal or nasal short binasal prongs including Argyle, Hudson, and INCA prongs long nasopharyngeal prongs |
• statistically & clinically significantly lower rate of re-intubation by using short binasal prong than the nasopharyngeal prong (RR0.63, CI: 0.40-0.97)
• no statistically significant difference for rates of death (RR1.68, CI:0.30-9.58), chronic lung disease (RR0.80, CI: 0.54-1.18), intraventricular haemorrhage (RR 0.168 CI: 0.30- 9.58), retinopathy of prematurity, sepsis or feeding intolerance among short single nasal and binasal prong • significantly higher incidence of nasal hyperaemia for using Argyle prong than Hudson prong (RR 2.39, 95% CI: 1.27-4.50), and Argyle prong reached statistically significant difference in incidence of hyperaemia for infants of the weight group ≤1000g, but no significant difference in the incidence of nasal bleeding & no cases of nasal septum necrosis for both interfaces |
no blinding in the studies |
| Fischer, et al., (2010) IIIB | Cohort study | 989 infants at mean GA 34 weeks, of mean BW 2142g a NICU in Switzerland |
provided NIV via nasal prongs or mask for infants with respiratory distress • Nasal prongs and mask were used alternatively every 4- 6 hour in every infant • Infants were positioned supine, prone or on their sides • observation of nose every 30-60 min & devices was removed every 2-4 hour for closer local inspection for any nasal trauma & to rate staging of injury by NPUAP • gentle massage without any ointment over pressure points of different nasal devices was performed every 2-4 hour • if presence of nasal trauma, massage with ointment (dexpanthenolum) & paracetamol for elevated pain score were provided to the infants • hydrocolloid film was placed between pressure points & the devices when increase in severity of the trauma |
• 42.5% of patients developed a nasal trauma of staging: stage I: 88.3% stage II: 11% stage III: 0.7% • correlation between nasal trauma due to NCAP and infant’s gestation age: 90% was < 28-week gestation 77% was < 32 weeks of gestation 28% was ≥ 32 weeks of gestation 11% was term baby - correlation between nasal trauma due to NCAP and infant’s gestation age & weight: • gestational age and birth weight were significantly inversely correlated with the severity of nasal trauma (p <0.001) • risk for development of nasal trauma was significantly increased when gestational age was < 32 weeks • other significant risk factors included duration of NCPAP and NICU stay (19days of NICU stay without trauma, 33 days with stage I trauma, 37 days with stage II-II trauma, p=0.001) |
reliability and validity on the modified rating system from US National Pressure Ulcer Advisory Panel was not mentioned |
| Fujii, et al. (2010) IIIB | Cohort study | 81 infants at GA ranged from less than 30 to over 39 weeks, of mean BW 1745g two NICUs in Tokyo, Japan |
• used Braden Q score to perform daily skin examination • limited number of position changes • nursed in incubator |
• most common location for the pressure ulcer was on the nose for infants using NCPAP (50%) • risk factors were significant at p<0.05 for birthweight, skin texture, incubator temperature, incubator humidity, support surface, limited number of position change & use of endotracheal intubation |
no blinding for the investigator |
| Goel, et al., (2015) IC | RCT | 118 infants of GA at 27 to 34 weeks a NICU in India |
used nasal mask or nasal prongs for bubble NCPAP • nasal toilet was provided every 4-hour • nasal trauma was evaluated in each shift daily • a large bore oro-gastric tube was inserted & open to atmosphere in vertical position to relieve stomach distension |
• significantly difference for failure between nasal mask group (13%) and prongs group (25%), p=0.15 • for severity of nasal trauma significantly difference for overall nasal trauma: 36% in mask group & 58% in prong group, p=0.02 significantly difference for moderate trauma 6.5% in mask group & 21% in prong group, p=0.03 significant lower incidence of overall nasal trauma in mask group • both groups were similar in terms of mild (p=0.55) & severe nasal trauma (p=0.48) |
• no blinding in the study • sample size might be underpowered to detect the difference between groups • single centre study |
| Haesler, E. (2017) IIIB | JBI Systematic review | 27 studies of RCT, quasi-experimental study, cohort study, expert opinion & bench research | • used prophylactic dressings to prevent medical device related pressure injuries (MDRPI) • nasal prongs for preterm infant |
• selected a correctly fitted & sized medical device made from the least damaging materials (Grade B) • conduct regular skin assessments under & around medical devices at least twice daily (Grade A) • regularly moisturize the skin underneath a MDRPI (Grade B, Level 1 evidence) • reposition medical devices on a regular basis whenever possible (Grade A, Leve 5 evidence) • alternating devices (Level 1 evidence) • Apply a prophylactic dressing underneath a medical device for infants (OR 3.43, p < 0.05) (Grade B, Level 1, 2 & 4 evidence) |
N/A |
| Imbulana, et al., (2018) IIIB | Systematic review | 13 RCTs
12 cohort studies
8 case studies
11 reviews 3930 infants of GA 23 to 37 weeks, BW at 530 to 2500g |
provided NIV via different nasal interface including binasal prongs, high flow (HF) cannula
• included different strategies to prevent nasal injury alternate HFNC with other NIV alternating nasal interfaces every 4-6 hours use of ointments use of a nasal barrier dressing, hydrocolloid dressing, during CPAP |
• common risk factors for nasal injury included the type of binasal prongs, incorrect sizing, and positioning of the prongs • skin immaturity, incubator humidity and temperature, and number of position changes were significant risk factors for pressure related injury • onset of nasal injury to the columella was reported as early as 18 hours to a mean of 2-3 days since starting CPAP • severe intranasal effects associated with CPAP including ulceration, granulation, & vestibular stenosis were reported within 9 days of CPAP commencement |
N/A |
| Kieran, et al., (2012) IB | RCT | 120 infants of GA less than 31 weeks, BW 888 to 1220g a NICU in Dublin, Ireland |
delivered NCPAP via nasal mask or binasal prong | • small proportion of infants were found nasal trauma, & equal occurrence of nasal trauma in two interfaces (3% in nasal prong, 3% in mask, p=.0946)
• statistically significant differences for re-intubated of infants < 28week between prongs (57%) & mask (22%) (p=0.11) • common injured areas: at the junction of the base of nasal septum & philtrum for mask; at the medial aspect of the nasal septum & the columella for prongs |
no blinding for the invention |
| Newnam, et al., (2013) IIIB | Systematic review | 46 studies of RCT, cohort study, case studies 989 infants of BW more than 800 to less than 3000g Level II or |
use nasal prong or masks in providing NIV to infants • recommended interventions in the discussions use of barriers, e.g. silicone gel sheet under the device to protect the nasal columella, and other pressure areas wetting the prongs with sterile water or saline to prevent friction during placement shaping prongs posterior to best align with the physiological angle of the neonate nares frequent assessment & examinations to identify hyperaemia early optimal developmental body positioning alternating the nasal mask and prongs to alter pressure points on nares & nasal mucosa avoidance of unnecessary suctioning adequate humidification |
• associated risk factors to increase incident of injury were identified as: smaller birth weight & lower gestational ages increased time/ hospital stays with NCPAP (significant) low APGAR scores (p=0.02 for score at 1 & p=0.06 for score at 5) pressure from the nasal prongs or air trauma from constant flow against soft nasal mucosa inappropriate nasal prong size |
• searching strategies was not mentioned • CI & effect of treatment were not stated in some studies |
| Newnam, et al., (2015) IC | RCT | 78 infants (36 vs 21 vs 22 in three study groups), at mean GA
26.77 weeks, of mean BW 873.36g a NICU of 70 Level III beds in southeastern USA |
provided NIV via continuous nasal prongs continuous nasal mask alternating mask/prongs every 4 hours • initial skin assessment done within 8 hours of extubation & at intervals of every 10-12 hours during receiving NIV • The Neonatal Skin Condition Scale (NSCS) was used to indicate dryness, erythema and breakdown or excoriation (graded 1 through 3) of skin injury in the study |
• significant differences for more less frequent and severe of erythema (p<0.001) & excoriation (p=0.007) in the rotation group than other 2 groups | • a convenience sampling method in a single centre • numbers of subject recruited for some groups below the target (24 each) |
| Razak, A. (2018) IIB | Systematic reviews of 12 studies of RCT, reviews | NA | used nasal mask and binasal prongs for NCPAP | • borderline reduction in nasal injury for mask group (RR0.80, 95% CI:0.64- 1.00, p=0.05) • occurrence of moderate to severe nasal trauma in preterm infants: 12% in nasal mask group, 24% in prong group (RR0.50, 95% CI: 0.31-0.77 not clearly listed out all included studies |
not clearly listed out all included studies |
| Say, et al., (2016) IB | RCT | 149 infants at GA 27 to 31 weeks, of BW 900 to 1600 g a NICU in Turkey |
provided NCPAP with nasal mask or nasal prongs
• perform daily examination for nasal trauma, hyperaemia, crusting, bleeding, excoriation & nasal passages |
• 13.5% of subjects were found with skin breakdown but no significant difference in skin breakdown in nasal prong (15%) and nasal mask (10%) (p=0.35)
• no significant differences found in other morbidities: necrotizing enterocolitis: p=0.61, 1% in nasal prong, 3% in mask spontaneous intestinal perforation: p=0.27, 1% in prong & 0% in mask duration of hospitalization: p=0.36, 18 days in prong & 25 days in mask (25 days) time for full feed: p=0.28, 13 days in prong & 14 days in mask |
no blinding in the study |
| Xie, 2014 IC | RCT | 65 infants (33 vs 32 in two study groups) at mean GA 32.6 weeks a NICU in China |
Provide NIV via prong & compare two methods for prevent nasal injury related to NIV apply paraffin oil around nostrils before inserting the prongs use a layer of hydrocolloid dressing to cover the infant’s nostrils surface (with a size of 2-3 cm cutting two holes adapted to the nose and nostrils) • Nostrils were inspected daily until NIV was off • Alternate the nasal prongs with mask every 6 hours for mild or moderate trauma was detected |
• 13.8% of nasal injury was found & significantly difference between 2 groups (p=0.01) • 6% for the group with hydrocolloid dressing • 21.8% for the group using the paraffin oil |
• sample size calculation was not clearly stated • demographic data for both groups was not listed out • the reliability of the instruments used to assess the skin injury was not mentioned |
| Yong, et al., (2005) IB | RCT | 89 infants (41 vs 48 in two study groups) at GA 28.7 to 29.7
weeks, of BW 1085 to 1105g a NICU in Kuala Lumpur Malaysia |
provided NCPAP via nasal prong or nasal mask | • no significant difference in the proportions for nasal trauma between 2 groups (p=0.5) • for nasal mask, injuries except narrowing of the passage were observed at the base of the nasal septum, at the junction between the philtrum & the base of the nasal septum • for the nasal prong, injuries were also similar but commonly at the medial aspect of the nostrils at the nasal septum - infants with nasal trauma had significantly lower mean birth weight & longer mean duration of NCPAP treatment (OR 1.04, 95% CI: 1.01- 1.07, p=0.003) |
• no blinding in the study |
| Guideline Evidence level & quality | Intervention | Limitations |
|---|---|---|
| The Royal Children’s Hospital, Melbourne: Clinical guideline (Nursing) (2018) - Continuous positive airway pressure (CPAP) - care in the newborn intensive care unit (Butterfly ward) IVC |
• choice of nasal interface binasal prongs nasal mask alternate between two interfaces to avoid trauma & attenuation of pressure • nursing care ensure correct selection of the size of the hat/prongs prongs should be positioned at least 2mm from the septum to avoid pressure alternating (“cycling”) between binasal prongs & mask every 4-6 hour mask should sit comfortably around the neonate’s nose without occluding the nostrils or touch the septum or over the lip or the eyes ensure well supported to the ventilator tubing to prevent drag on the nasal interface |
• not clear evidence searching method was stated • details appraisal on the evidence were not stated |
| Queensland Health: Queensland Clinical Guidelines: Maternity and Neonatal clinical guideline: neonatal respiratory distress including CPAP (2014) IVC | • choice of nasal interface binasal prongs or nasal mask • measures to prevent pressure injury correct selection of size &/or device type position binasal prongs 2mm from the nares & not in contact with the septal columella prongs should fit nares firmly without blanching skin correct positioned of the interface: not distorting features or pushing nasal structure upwards check septal columellar integrity, and eyes for clearly visible inspect for nasal redness, skin breakdown, bruising, indentation, bleeding, altered nasal shape ears for pressure areas, creases, or folds forehead for using midline device (prongs or mask) nasal bridge mid-facial indentation (mask) remove hat to inspect head with cares empty accumulated condensation in the tubing to prevent aspiration securing device not causing indentation, pitting or periorbital oedema position the infant to avoid inadvertent tension to the interface &/or accumulation of condensate at the nares insertion of an orogastric tube on free drainage or regular aspiration 4-6 hourly to relieve abdominal distension for infants with tube feeding, free drainage the tube half hour after feed • use pacifier or chin strap to close the mouth to achieve optimal pressure of NIV |
• not clear searching method was stated • details of the evidence and the appraisal were stated |
Conclusions
Implication for practice
The evidence-based clinical practice guideline can be preliminary developed according to the findings of the 16 studies. Details of the interventions will be further discussed in the workgroup to evaluate the feasibility and practical of interventions in each core element. To streamline the workflow in daily operation will be the priority for consideration. After finalized the guideline, staff training on the practice will be commenced to facilitate the adaption of the change. As stated in many studies that vigilance nursing cares is the successful factor in promotion comfort and prevention of complications on the infants receiving NIV [13, 51]. Afterwards, the guideline will be implemented in a local NICU of 23 level III care beds, and evaluation on the effects of infant’s comfort including time of sleeping or quiet state, pain level; and complications associated with NIV including occurrence and onset of the nasal injury, and change of abdominal girth will be assessed. In addition, nurse’s knowledge and compliance on the practice will be assessed by pre and post NIV knowledge tests and audit on NIV care bundle, respectively.
Practice developed from evidence-based strengthens service quality and standard that allows benchmarking with other institutions. Moreover, it provides rationale to support each intervention, and facilitates healthcare providers to explore more evidence to improve current practice. On the other hand, evidence are continuously discovered and updated according to empirical studies in the clinical areas. Thus, every clinical practice guideline should be regularly reviewed and refined accordingly.
References
1. Berger TM, Fontana M, Stocker M. The journey towards lung protective respiratory support in preterm neonates. Neonatol. 2013;104: 265-274.
2. Courtney SE, Barrington KJ. Continuous positive airway pressure and noninvasive ventilation. Clin Perinatol. 2007; 73-92.
3. Fathi O, Schlegel AB, Shepherd EG. Non-invasive ventilation of the neonate. Intech Open. 2017.
4. Pillow JJ. Which continuous positive airway pressure system is best for the preterm infant with respiratory distress syndrome? Clin Perinatol. 2012; 39: 483-496.
5. American Academy of Pediatrics (AAP). Respiratory support in preterm infants at birth. Pediatrics. 2014; 133(1): 171-174.
6. Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatol. 2019; 115: 432-450.
7. Komatsu DFR, Diniz EMA, Ferrano AA, et al. Randomized controlled trial comparing nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure in premature infants after tracheal extubation. Revista da Associação Médica Brasileira. 2-16; 62(6): 568-574.
8. Lemyre B, Davis PG, De Paoli AG, et al. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation (Review). Cochrane Database of Sys Rev. 2017; (2).
9. Meneses J, Bhandari V, Alves JG, et al. Nonivasive ventilation for respiratory distress syndrome: A randomized controlled trial. Pedia. 127(2): 300-307.
10. Mulder EEM, Lopriore E, Rijken M, et al. Changes in respiratory support of preterm infants in the last decade: Are we improving? Neonatol. 2011; 101:247-253.
11. Salvo V, Lista G, Lupo E, et al. Noninvasive ventilation strategies for early treatment of RDS in preterm infants: An RCT. Pediatr. 2015; 135(3): 444-451.
12. Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants (Review). Cochrane Database of Systematic Reviews. 2016; (6).
13. Bober K, Swietlinski J, Zejda J, et al. A multicenter randomized controlled trial comparing effectiveness of two nasal continuous positive airway pressure devices in very-low-birth-weight infants. Pediatr Crit Care Med. 2012; 13(2): 191-196.
14. Campbell E, Pacifico MD. Columella erosion secondary to nasal prongs in the neonate. BMJ. 2016.
15. Fischer C, Bertelle V, Hohlfeld J, et al. Nasal trauma due to continuous positive airway pressure in neonates. Arch Dis Child Fetal Neonatal Ed. 2010; 95(6): F447-51.
16. Imbulana DI, Manley BJ, Dawson JA, et al. Nasal injury in preterm infants receiving non-invasive respiratory support: A systematic review. Arch Dis Child Fetal Neonatal Ed. 2018; 103(1): F29-F35.
17. Hogeling M, Fardin SR, Frieden HJ, et al. Forehead pressure necrosis in neonates following continuous positive airway pressure. Pediatric Dermatol. 29(1): 45-48.
18. Mathai SS, Rajeev A, Adhikari KM. Safety and effectiveness of bubble continuous positive airway pressure in preterm neonates with respiratory distress. Med J Armed Forces Ind. 2014; 70: 327-331.
19. Tagare A, Kadam S, Vaidya U, et al. A pilot study of comparison of BCPAP vs. VCPAP in preterm infants with early onset respiratory distress. J Tropical Pediatr. 2010; 56(3): 191-194.
20. Buckwalter KC, Cullen L, Hanrahan K, et al. Iowa Model of evidence-based practice: revisions and validation. Worldviews on Evidence-Based Nurs. 2017; 14(3): 175-182.
21. The Johns Hopkins University. Johns Hopkins nursing evidence-based practice, 2017.
22. De Paoli AG, Davis PG, Faber B, Morley CJ. (2008). Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No.: CD002977. DOI: 10.1002/14651858.CD002977.pub2.
23. Goel S, Mondkar J, Panchal H, et al. (2015). Nasal mask versus nasal prongs for delivering nasal continuous positive airway pressure in preterm infants with respiratory distress: A randomized controlled trial. Ind Pediatr. 2015; 52(12): 1035-1040.
24. Razak A. Should nasal mask or binasal prongs be used for continuous positive airway pressure in preterm infants? Archives of Disease in Childhood. 2018; 10.1136/archdischild-2017-314695.
25. Chandrasekaran A, Thukral A, Jeeva Sankar M, et al. Nasal masks or binasal prongs for delivering continuous positive airway pressure in preterm neonates—a randomised trial. Eur J Pediatr. 2017; 176(3): 379-386.
26. Kieran EA, Twomey AR, Molloy EJ, et al. Randomized trial of prongs or mask for nasal continuous positive airway pressure in preterm infants. Pediatr. 2012; 130(5): e1170-e1176.
27. Say B, Kanmaz Kutman H, Oguz S, et al. Binasal Prong versus Nasal Mask for Applying CPAP to Preterm Infants: A Randomized Controlled Trial. NeonatoL. 2016; 109(4): 258-64.
28. Yong SC, Chen SJ, Boo NY. Incidence of nasal trauma associated with nasal prong versus nasal mask during continuous positive airway pressure treatment in very low birthweight infants: a randomized control study. Arch Dis Childh- Fetal Neonatal Ed. 2005; 90(6),: F480-F483.
29. Newnam K, McGrath J, Estes T, et al. An Integrative Review of Skin Breakdown in the Preterm Infant Associated with Nasal Continuous Positive Airway Pressure. J Obstet Gynecol Neonatal Nurs. 2013; 42(5): 508-516.
30. Xie LH. Hydrocolloid dressing in preventing nasal trauma secondary to nasal continuous positive airway pressure in preterm infants. World J Emerg Med. 2014; 5(3): 218-222.
31. August DL, New K, Ray RA, et al. Frequency, location and risk factors of neonatal skin injuries from mechanical forces of pressure, friction, shear and stripping: A systematic literature review. J Neonatal Nurs. 2018; 24: 173-180.
32. Haesler E. Pressure injuries: Preventing medical device related pressure injuries. JBI Evidence Summaries, AN: JBI18873, 2017.
33. Newnam KM, McGrath JM, Salyer J, et al. A comparative effectiveness study of continuous positive airway pressure-related skin breakdown when using different nasal interfaces in the extremely low birth weight neonate. App Nurs Res. 2015; 28: 36-41.
34. Fujii K, Sugam J, Okuwa M, et al. Incidence and risk factors of pressure ulcers in seven neonatal intensive care units in Japan: a multisite prospective cohort study. Int Wound J. 2010; 7(5): 323-328.
35. Brunherotti MAA, Martinex FE. Influence of body position on the displacement of nasal prongs in preterm newborns receiving continuous positive airway pressure. Revista Paulista De Pediatria. 2015; 33(3): 28-285.
36. McCoskey L. Nursing care guidelines for prevention of nasal breakdown in neonates receiving nasal CPAP. Advances in Neonatal Care. 2008; 8(2): 116-124.
37. Flanagan KA. Noninvasive ventilation in premature neonates. Advances in Neonatal Care. 2016; 16(2): 91-98.
38. Nascimenta RM, Ferreira ALC, Coutinho ACFP, et al. The frequency of nasal injury in newborns due to the use of continuous positive airway pressure with prongs. Rev. Latino-am Enfermagem. 2003; 17(4): 489-494.
39. Badr LK, Zeineddine MH, Abbas H, et al. Neoseal to prevent nasal injury in preterm infants receiving oxygen therapy. Neonatal Network. 2016; 35(4), 228-233.
40. Sorensen D, Frederiksen K, Grofte T, et al. Practical wisdom: A qualitative study of the care and management of non-invasive ventilation patients by experienced intensive care nurses. Intensive Crit Care Nur. 2013; 29: 174-181.
41. Chawla D. Optimizing nasal interface for continuous positive airway pressure in neonates. Ind Pediatr. 2015; 52: 1027-1028.
42. Bishopp A, Oakes A, Pitterson PA, et al. The preventative effect of hydrocolooid dressings on nasal bridge pressure ulceration in acute non-invasive ventilation. The Ulster Medical J. 2019; 88(1): 17-20.
43. Cai JY, Zha ML, Chen HL. Use of a hydrocolloid dressing in the prevention of device-related pressure ulcers during non-invasive ventilation: A meta-analysis of randomized controlled trials. Wound Manage Prev. 2019; 65(2): 30-38.
44. Gillies D, Wells D, Bhandari AP. Positioning for acute respiratory distress in hospitalized infants and children (review). Cochrane Database Syst Rev. 2012; 7.
45. Fernandez RM, Roquei FM, Izquierdo DA, et al. Infant position in neonates receiving mechanical ventilation (review). Cochrane Database of Systematic Reviews. 2016; 11.
46. Baharestani MM, Ratliff CR. Pressure ulcers in neonates and children: An NPUAP while paper. Adv Wound Care. 2007; 20(4): 208-220.
47. Bushell T, McHugh C, Meyer MP. A comparison of two nasal continuous positive airway pressure interfaces-a randomized crossover study. J Perinat Med. 2013; 6: 53-59.
48. Lissauer T, Duke T, Mellor K, et al. Nasal CPAP for neonatal respiratory support in low and middle-income countries. Arch Dis Child Fetal Neonatal Ed. 2018; 102(3): F194-F196.
49. Raurell-Torreda M, Romero-Collado A, Rodriguez-Palma M, et al. Prevention and treatment of skin lesions associated with non-invasive mechanical ventilation. Recommendations of experts. Enfermeria Intensiva. 2017; 28(1): 31-41.
50. Murray JS, Noonan C, Quigley S, et al. (2013). Medical device-related hospital-acquired pressure ulcers in children: An integrative review. J Pediatr Nurs. 2013; 28: 585-595.
51. Swietlinski J, Bober K, Gajewska E, et al. Introduction of infant flow nasal continuous airway pressure as the standard of practice in Poland: The initial 2-year experience. Pediatr Critical Care. 2007; 8(2): 109-114.
52. The Royal Children’s Hospital Melbourne. Clinical guidelines (nursing): continuous positive airway pressure (CPAP) - care in the newborn intensive care unit (butterfly ward), 2018.
Received:December 03, 2020;
Accepted:December 11, 2020;
Published:December 25, 2020.
To cite this article : Chan SY, Chau JPC. Evidence-Based Practice for Care of Preterm Infants Receiving Non-Invasive Ventilation. European Journal of Respiratory Medicine. 2021; 3:1.
© 2020 Chan SY,et al.
