Research article/ Open Access
DOI:10.31488/EJRM.150
Hyperbaric Oxygen Therapy Decreases Chronic Low-Grade Inflammation, Evaluate by suPAR Test
Garber MG*1,2,4, Pedrero F3,4, Sorge V2,3
1. University hospital La Zarzuela, Madrid, Spain
2. Vidafull Hyperbaric center, Naples,USA
3. Clinica Castello 68, Madrid, Spain
4. Reyou Suisse Clinic, Switzerland
*Corresponding author: : Dr. Miguel G. Garber, University hospital La Zarzuela, Madrid, Spain
Abstract
The aim of this study was to investigate the effects of hyperbaric oxygen therapy (HBOT) on Systemic low grade of chronic inflammation (SLGCI), HBOT may be used as a main or adjuvant treatment for inflammation, leading to the main aim of this study, which was to verify the applicability of HBOT as a safe and tolerable tool in healthy person with SLGI. More scientific studies show that low grade of chronic inflammation (SLGCI), a phenomenon often referred to as “inflammaging” has a significant impact on the aging process, is clear that reducing or preventing SLGCI, plays a crucial role in overall health-spam. The soluble urokinase plasminogen activator receptor (suPAR) is associated with SLGCI and is used as a prognostic factor of inflammation progression. This study was using a suPAR test to evaluate whether HBOT may reduce SLGCI. The conclusion: there was a significant decrease in the value of suPAR test by -37.30%±33.04 post-HBOT (P<0.0001) showed that HBOT is a good tool for improving Systemic low grade of chronic inflammation.
Background
Low grade of chronic inflammation (SLGCI), a phenomenon often referred to as “inflammaging” has a significant impact on the aging process, is clear that reducing or preventing SLGCI, plays a crucial role in overall health-spam. Although the association between inflammation and chronic conditions is widely recognized [1], Inflammation acts as both a ‘friend and killer’: it is an essential component of host defense and immunosurveillance, yet a chronic low-grade inflammatory state is a pathological feature of a wide range of chronic conditions, such as the metabolic syndrome (MetS), non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD) and others [2-5]. Inflammation is an active process involving cytokines and other anti-inflammatory mediators, particularly lipids, peptides, cytokines, and others.
Evidence is emerging that the risk of developing chronic inflammation can be traced back to early development, and its effects are now known to persist throughout the life span to affect adulthood health and risk of mortality [6-8].
Characteristic of SLGCI as a trigger can be lifestyle, exposome, metabolic dysfunction, tissue damage, stress, is chronic (persistent no resolved, low grade in level, with collateral damage, related to age [9] and the more sensible biomarker is soluble urokinase plasminogen activator receptor (suPAR) [10-11-13,19]
Hyperbaric oxygen therapy (HBOT) is based on the administration of approximately 100% oxygen inside a chamber pressurized to more than 1.4 Atmospheres Absolute (ATA). This therapy is used as a main or adjuvant treatment for inflammation, chronic wounds, ischemia, and infections [14], hyperbaric oxygen treatment could mitigate oxidative insults in endothelial cell [15]. Hyperoxia increases the amount of dissolved oxygen in blood and plasma with the subsequent action on tissue oxygenation and mitochondrial metabolism [16]. These mechanisms explain why oxygen is used as a therapy to optimize oxygen transport capacity, Hyperbaric oxygen treatment significantly decreased inflammation and was associated with HBOT-induced oxidative stress reduces the concentrations of pro-inflammatory acute phase proteins (TNF-alpha, IL-1 beta and IL-6 expression), interleukins and cytokines and increases growth factors and other pro-angiogenesis cytokines [17-18].
Soluble urokinase-type plasminogen activator receptor (suPAR) is a chronic low grade inflammation marker associated with the development of a range of degenerative diseases, including cancer, neurodegenerative, and cardiovascular disease [21]. Today suPAR plasma levels has a potential role in associated diseases, as well as the underlying mechanisms and give a prognostic value as a unique marker of chronic low grade inflammation [22], Soluble urokinase plasminogen activator receptor (suPAR) is a protein derived from the cleavage and release of the cell membrane-bound urokinase plasminogen activator receptor (uPAR), which is part of the plasminogen activation system and implicated in pathological processes of inflammation, proteolysis, tissue remodeling, and cancer metastasis [23,24].
Methods
This prospective cohort study included 12 healthy males who showed two or more parameters of SLGI.
Study population
After agreement from the local and central institutional research ethics committees (F20240527), this prospective interventional study was performed over a 6-month period in the hyperbaric medicine center at Naples (US) Vidafull, all methods were performed in accordance with the relevant guidelines and regulations. After signed informed consent, 12 patients were assigned to HBOT. Measurement points were evaluated at baseline, half-point of the treatment protocol (10th session), the day of the last HBOT session and 1week after the HBOT. Participants, characteristics and demographic variables are reported in Table 1. Participants were required to be generally healthy to be eligible and be included in the current study (N = 12) did not differ significantly from other participants healthy at age 65. During the study period, data were collected from 12 healthy volunteers, patients with a chronic low-grade inflammation with suPAR positive (overall age range 55 to 88 years) for the first HBOT session.
Table 1.
| Male | 6 |
| Female | 6 |
| Age | 65-72 |
| Height | 162-181 |
| Weight | 56-92 |
| Non-smokers, (%) | 0 (100%) |
| Body mass index (kg/m2) | 28.5 ± 5.8 |
| Anti-inflammatory medication use, n (%) | 0 (0%) |
| CRP (mg/L) | 2.7 ± 5.6 |
| IL-6 (pg/mL) | 2.1 ± 2.6 |
| suPAR (ng/mL) | 4.0 ± 1.0 |
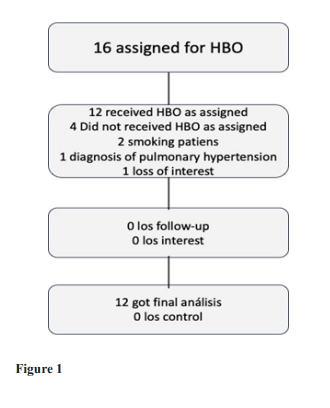
Figure 1:
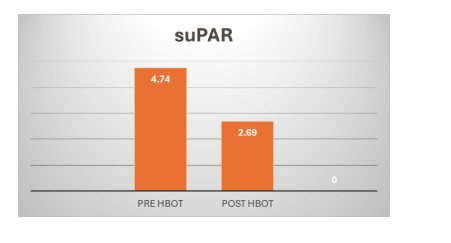
Figure 2:
Sixteen individuals were assigned to HBOT. Four patients did not complete baseline assessments and were excluded. All 12 patients who completed baseline evaluations completed the interventions. The baseline of the cohorts following exclusion of the patients are provided in table1.
Upon enrolment, participants provided written informed consent, whole blood, plasma, and answered a comprehensive questionnaire, including items on height, weight and smoking status. total patients were investigated in this group for 15 sessions of HBOT.
The patients with inflammatory conditions were prescribed HBOT for wellness protocol: consisted of breathing 100% oxygen at 2.2 atmosphere absolute (ATA) for 70 min of iso-pression, with 2 breaks of 5 minutes breathing air (21% of oxygen) This is called the hyperoxic-hypoxic paradox (HHP) [20]. Blood samples were collected before HBOT treatment (BT), and after HBOT treatment (AT), Chronic inflammation markers measurement were assessed SLGCI using biomarkers, suPAR. Plasma suPAR (ng/mL). The suPARnostic AUTO Flex ELISA (ViroGates A/S, Birkerød, Denmark) according to the manufacturer’s instructions. The detection limit of the assay was 0.1 ng/mL. The intraassay correlation of repeat measurements of the same sample was r = 0.98 and coefficient of variation (CV) = 2.4%, and the interassay correlation was r = 0.81 and CV = 12.8%. suPAR levels were correlated at age 38 and 45, r = .58, p < .001, and increased on average 0.6 ng/mL from age 38 to 45, with changes ranging from −5.4 to 9.3 ng/mL.
Statistical analysis
Continuous data were expressed as means ± standard-deviation. The normal distribution for all variables was tested using the Kolmogorov-Smirnov test.
Categorical data is expressed in numbers and percentages and compared by chi-square tests. Univariate analyses were performed using Chi-Square/Fisher’s exact test to identify significant variables (P<0.05).
To evaluate HBOT’s effects, a repeated measures ANOVA model was used to test the main within-subject effect. Post hoc tests on the means were conducted to test for time differences using t tests with a Bonferroni correction, as recommended for statistic specialist.
Results
Compared to the baseline, the test result for low grade chronic inflammation suPAR were significantly decrease at the 15th session and post-HBOT by 4.74±0.28, decrease to 2,69±0.42 respectively P value is less than 0.01
Overall, these statistics demonstrate that the assay is both sensitive and precise for measuring suPAR levels, making it a valuable tool in both clinical and research contexts. However, attention should be given to interassay variability, and further investigation may be warranted to understand the factors influencing changes in suPAR levels over time.
Conclusion
The study provides preliminary evidence that HBOT may be effective in reducing SLGCI, as measured by suPAR levels. However, the small sample size, lack of control group, and limited discussion of long-term outcomes and underlying mechanisms warrant further investigation.
Future research should address these limitations and explore the potential of HBOT as a safe and tolerable tool for improving inflammation in healthy individuals.
Reference
1. Calder PC, Ahluwalia N, Albers R, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr. 2013; 109: S1-S34.
2. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444: 860-867.
3. Libby P. Inflammation in atherosclerosis. Nature. 2002; 420: 868-874
4. Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation, an integrated view. EMBO Mol Med. 2013; 5: 661-674.
5. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019; 25, 1822-1832.
6. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011; 137: 959–997.
7. Fleming TP, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018; 391: 1842-1852.
8. Renz H, et al. An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol. 2017; 140, 24-40.
9. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018; 128: 2657-2669.
10. Rasmussen LJH, Petersen JEV, Eugen-Olsen J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front Immunol. 2021 Dec 2;12:780641. doi: 10.3389/fimmu.2021.780641.
11. Enocsson H, Wetterö J, Skogh T, Sjöwall C. Soluble urokinase plasminogen activator receptor levels reflect organ damage in systemic lupus erythematosus. Transl Res. 2013 Nov;162(5):287-96. doi: 10.1016/j.trsl.2013.07.003. Epub 2013 Aug 1.
12. Füller D, Liu C, Ko YA, Alkhoder AA, Desai SR, Almuwaqqat Z, et al. Soluble urokinase Plasminogen Activator Receptor (suPAR) mediates the effect of a lower education level on adverse outcomes in patients with coronary artery disease. Eur J Prev Cardiol. 2024 Mar 27;31(5):521-528. doi: 10.1093/eurjpc/zwad311.
13. Hamie L, Daoud G, Nemer G, Nammour T, El Chediak A, Uthman IW, et al. SuPAR, an emerging biomarker in kidney and inflammatory diseases. Postgrad Med J. 2018 Sep;94(1115):517-524. doi: 10.1136/postgradmedj-2018-135839. Epub 2018 Sep 3.
14. Takahashi M, Iwatsuki N, Ono K, Tajima T, Akama M, Koga Y. Hyperbaric oxygen therapy accelerates neurologic recovery after 15-minute complete global cerebral ischemia in dogs. Crit Care Med. 1992;20:1588–1594.
15. Godman CA, Joshi R, Giardina C, Perdrizet G, Hightower LE. Hyperbaric oxygen treatment induces antioxidant gene expression. Ann N Y Acad Sci. 2010 Jun;1197:178-83. doi: 10.1111/j.1749-6632.2009.05393.x.
16. Daugherty W P, Levasseur JE, Sun D, Rockswold GL, Bullock MR. Effects of hyperbaric oxygen therapy on cerebral oxygenation and mitochondrial function following moderate lateral fluid-percussion injury in rats. Journal of Neurosurgery. 2004;101(3):499–504.
17. Desmarquest P, Chadelat K, Corroyer S, Cazals V, Clement A. Effect of hyperoxia on human macrophage cytokine response. Respir Med. 1998 Jul;92(7):951-60.
18. Wilson HD, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Res. 2006 Jul 7;1098(1):126-8. doi: 10.1016/j.brainres.2006.04.088. Epub 2006 Jun 5.
19. Dowsett J, Ferkingstad E, Rasmussen LJH, et al. Eleven genomic loci affect plasma levels of chronic inflammation marker soluble urokinase-type plasminogen activator receptor. Commun Biol. 2021; 4, 655.
20. Hadanny A, Efrati S. The Hyperoxic-Hypoxic Paradox. Biomolecules. 2020 Jun 25;10(6):958. doi: 10.3390/biom10060958.
21. Dowsett J, Ferkingstad E, Rasmussen LJH, et al. Eleven genomic loci affect plasma levels of chronic inflammation marker soluble urokinase-type plasminogen activator receptor. Commun Biol. 2021; 4, 655.
22. Fei Huang, Yueqiang Li, Ranran Xu, Anying Cheng, Yongman Lv, et al. The Plasma Soluble Urokinase Plasminogen Activator Receptor Is Related to Disease Activity of Patients with ANCA‐Associated Vasculitis. Mediators of Inflammation. 2020; 10.1155/2020/7850179.
23. Smith HW, Marshall CJ. Regulation of cell signalling by uPAR, Nature Reviews Molecular Cell Biology. 2010;11, no. 1, 23–36
24. Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball, Disease Markers. 2009; 3: 157–172,
Received:June 10, 2024;
Accepted: July 22, 2024;
Published: July 29, 2024.
To cite this article : Garber MG, Pedrero F, Sorge V. Hyperbaric Oxygen Therapy Decreases Chronic Low-Grade Inflammation, Evaluate by Supar Test. European Journal of Respiratory Medicine. 2024; 6(3): 439 - 443. doi: 10.31488/EJRM.150.
© The Author(s) 2024
