Research article/ Open Access
DOI:10.31488/EJRM.143
Longitudinal Assessment of Real-World Treatments and Outcomes in Patients with Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Multicenter, Prospective, Observational Study
Mineo Katsumata 1,2, MD, PhD, Yusuke Inoue1*, MD, PhD, Shiro Imokawa3, MD, PhD, Naoki Koshimizu4, MD, Toshihiro Shirai5, MD, PhD, Mikio Toyoshima6, MD, PhD; Hideki Yasui1, MD, PhD, Masato Karayama1,7, MD, PhD, Yuzo Suzuki1, MD, PhD, Hironao Hozumi1, MD, PhD, Kazuki Furuhashi1, MD, PhD, Noriyuki Enomoto1, MD, PhD, Tomoyuki Fujisawa1, MD, PhD, Naoki Inui1,8, MD, PhD, Takafumi Suda1, MD, PhD
1. Second Division, Department of Internal Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Chuo-ku, Hamamatsu, Shizuoka 431-3192, Japan
2. Department of Pulmonary Medicine, Seirei Hamamatsu General Hospital, 2-12-12 Sumiyoshi, Chuo-ku, Hamamatsu, Shizuoka 430-8558, Japan
3. Department of Respiratory Medicine, Iwata City Hospital, 512-3 Ohkubo, Iwata, Shizuoka 438-8550, Japan
4. Department of Respiratory Medicine, Fujieda Municipal General Hospital, 4-1-11 Surugadai, Fujieda, Shizuoka 426-8677, Japan
5. Department of Respiratory Medicine, Shizuoka General Hospital, 4-27-1 Kita-ando, Shizuoka 420-8527, Japan
6. Department of Respiratory Medicine, Hamamatsu Rosai Hospital, 25 Shougen-cho, Chuo-ku, Hamamatsu, Shizuoka 430-8525, Japan
7. Department of Chemotherapy, Hamamatsu University School of Medicine, 1-20-1 Handayama, Chuo-ku, Hamamatsu, Shizuoka 431-3192, Japan
8. Department of Clinical Pharmacology and Therapeutics, Hamamatsu University School of Medicine, 1-20-1 Handayama, Chuo-ku, Hamamatsu, Shizuoka 431-3192, Japan
*Corresponding author: : Yusuke Inoue MD, PhD, Second Division, Department of Internal Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Chuo-ku, Hamamatsu, Shizuoka 431-3192, Japan, Tel: +81-53-435-2263; Fax: +81-53-435-2386
Abstract
Background: Despite its high incidence and significant influence on patient outcomes, there remains no established treatment strategy for rheumatoid arthritis (RA)-associated interstitial lung disease (ILD). Methods: We aimed to prospectively clarify the real-world treatment landscape for RA-ILD and to assess the efficacy and safety of treatments. Methods: This multicenter, prospective, observational study enrolled patients with RA-ILD who were scheduled to receive treatment for ILD between February 2016 and April 2020. The dyspnea scores, pulmonary function findings, and lung images on high-resolution computed tomography (HRCT) were longitudinally evaluated at 3, 6, and 12 months after initiation of treatment. The primary endpoint was the change in forced vital capacity (FVC) from baseline at month 12. A composite classification of outcomes was built on the changes in dyspnea score, FVC, and HRCT findings. Results: All 18 patients enrolled in the study were administered prednisolone as either monotherapy or combination therapy with tacrolimus and/or methylprednisolone pulse therapy. FVC at month 12 was improved or stabilized in six patients each, resulting in an improvement or stabilization rate of 67% (95% confidence interval, 41%–87%). Using the composite classification, disease improvement and stabilization were achieved in six (33%) and five (28%) patients at month 12, respectively. There were no life-threatening adverse events or treatment-related deaths. Conclusion: Corticosteroids play a major role in the treatment of RA-ILD and offer improvement or stabilization of FVC as well as the composite outcomes in approximately 60% of patients at month 12.
Keywords: rheumatoid arthritis, interstitial lung disease, treatment, outcome, corticosteroids
Introduction
Interstitial lung disease (ILD) is a critical and clinically relevant manifestation of connective tissue diseases (CTDs) with considerable effects on patients’ quality of life and survival outcomes[1]. Among the spectrum of CTDs, rheumatoid arthritis (RA), a systemic disease characterized by joint inflammation leading to destructive bone erosion, has a high prevalence of approximately 5 per 1,000 adults [2,3]. Importantly, RA is the most common CTD associated with ILD [4]. In patients with RA, ILD is observed on high-resolution computed tomography (HRCT) in up to 60% of cases [5], and is the second leading cause of death after cardiovascular disease [6].
Despite its high incidence and significant influence on patient outcomes, there remains no established treatment strategy for RA-ILD, mainly because of a lack of prospective, controlled trials comparing medications for RA-ILD [5]. Nevertheless, experts in the field have proposed treatment strategies for RA-ILD on the basis of retrospective studies and case series. For example, Yamakawa et al. [7] proposed the following management algorithm for progressive RA-ILD: for patients with high articular activity of RA, it is recommended to use disease-modifying anti-rheumatic drugs (DMARDs) followed by additional treatment with anti-inflammatory agents such as corticosteroids for the inflammation-dominant phenotype or an antifibrotic agent, nintedanib, for the fibrosis-dominant phenotype; for patients without such activity, anti-inflammatory agents or nintedanib are recommended according to the dominant phenotype. However, despite the proven efficacy of nintedanib for progressive fibrosing (PF) ILDs, including RA-ILD, in a pivotal phase 3 trial [8], there remains an extreme paucity of data on whether patients with RA-ILD derive benefit from anti-inflammatory drugs due to the lack of prospective studies. Consequently, the optimal therapeutic approach for RA-ILD remains debatable.
We conducted a multicenter, prospective, observational study to clarify the treatment landscape for RA-ILD in the era before nintedanib was approved for PF-ILDs and to assess the efficacy and safety of treatments for the disease in patients requiring intervention in a real-world setting.
Materials and Methods
Study design and participants
This multicenter, prospective, observational study was conducted at 5 institutions in Japan between February 2016 and April 2020. Eligible patients were aged ≥20 years and satisfied the 2010 American College of Rheumatology/European League Against Rheumatism RA classification criteria [9] as definite RA. Patients were also required to have ILD. Specifically, the enrolled patients were required to have bilateral reticular or ground-glass opacities compatible with ILD affecting ≥10% of the lung volume on HRCT diagnosed by board-certified pulmonologists and radiologists at each institution. Patients were also required to have progressive ILD, defined as ILD for which a physician judged medications to be necessary because of an increase in fibrosis, new bilateral ground-glass opacity or consolidation superimposed on a background pattern consistent with RA-ILD, or a relative decline in forced vital capacity (FVC) or pulmonary diffusion capacity of carbon monoxide (DLCO), with worsening of respiratory symptoms. Key exclusion criteria were pregnancy, active infection, lung deterioration that could be explained by different causes, and coexistence of other CTDs.
Study treatments and outcomes
Therapeutics for RA-ILD were not prespecified and were at the discretion of the physicians. All patients enrolled in the study underwent a baseline HRCT evaluation of the chest, pulmonary function tests including spirometry and DLCO, and a 6-minute walk distance test. The partial pressure of oxygen (PaO2) on arterial blood gas analyses and serum KL-6 level were measured. Dyspnea was assessed using the modified Medical Research Council (mMRC) dyspnea scale. The efficacy of therapeutics was evaluated by mMRC score calculation, spirometry, and HRCT at months 3, 6, and 12 after treatment initiation.
The primary endpoint was the change in FVC at month 12. The secondary endpoints were the changes in FVC at months 3 and 6, DLCO at months 3, 6, and 12, mMRC scores at months 3, 6, and 12, and radiographical severity on HRCT at months 3, 6, and 12. Improvement and progression of FVC and DLCO were defined as ≥10% increase or decrease from baseline, respectively [10,11]. Other findings were considered to reflect a stabilized condition. HRCT images with 1.0- or 1.5-mm-thick sections were evaluated by both board-certified diagnostic radiologists and pulmonologists at each institution as well as two pulmonologists as a central review without knowledge of the clinical data (MK, YI). The ILD patterns on HRCT were characterized as usual interstitial pneumonia (UIP) pattern, probable UIP pattern, indeterminate UIP pattern, or alternative diagnosis including non-specific interstitial pneumonia (NSIP) and organizing pneumonia (OP) based on the ATS/ERS/JRS/ALAT clinical practice guideline [12]. Determination of radiological improvement or progression of ILD was left to the discretion of individual investigators [10]. Acute exacerbation (AE) was diagnosed in accordance with the 2016 international working group report criteria for AE of idiopathic pulmonary fibrosis (IPF) [13] with slight modifications as described previously [14]. Briefly, AE-RA-ILD had to fulfill all four of the following criteria: (1) presence of fibrosing ILD on previous HRCT; (2) acute worsening or development of dyspnea within 1 month; (3) new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with fibrosing ILD on HRCT; and (4) deterioration not fully explained by cardiac failure or fluid overload.
For all variables, patients who had died from RA-ILD at the specified time point for assessment were classified as progressed.
Ethics
The study was approved by the local institutional review boards at Hamamatsu University School of Medicine (No. E15-201) and all other participating institutions. The study was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The study was registered in the UMIN Clinical Trials Registry as UMIN000020208. All patients provided written informed consent to participate in the study.
Statistical analysis
The sample size for the study was estimated on the assumption that the improvement or stabilization rate for FVC at month 12 would be approximately 50% [15] with a threshold of 20%. The study design was based on the Simon single-stage optimal design (80% power; α = 0.05; P0 = 0.20; P1 = 0.50). Assuming that 10% of patients would be ineligible for meeting the exclusion criteria, major protocol violations, and other reasons, the required sample size was determined at 20 patients. The Wilcoxon rank-sum test was used to compare the FVC values and mMRC scores between different time points. All statistical analyses were performed using EZR statistical software [16] version 1.55 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). A two-tailed value of P < 0.05 was considered statistically significant.
Results
Patients and treatments
A total of 18 Japanese RA patients who were scheduled to receive treatment for ILD at five institutions were enrolled in the study. The distributions of the baseline covariates and treatments are presented in Table 1. The median age was 72 years (range, 49–85) and most patients had dyspnea (mMRC score, 1–4). The median time since onset of RA was 1 year (mean, 4.5 years), and 6 (32%) patients were diagnosed with RA-ILD shortly after their diagnosis of RA (within 30 days). Most patients had impaired %DLCO (median, 54%; two cases had missing data), resulting in relatively low values of PaO2 (median, 72 Torr) and 6-minute walk distance (median, 345 m), while %FVC was relatively preserved (median, 80%). On HRCT, only one case each was classified as UIP pattern or probable UIP pattern, and NSIP pattern was most frequently observed (N = 8; 44%). Regarding baseline treatments for RA, 9 (50%) patients had received synthetic DMARDs (methotrexate, N = 1; salazosulfapyridine, N = 5; bucillamine, N = 1; tacrolimus, N = 2; iguratimod, N = 1). Three (17%) patients had received combination therapy with synthetic DMARD (salazosulfapyridine or tacrolimus) plus low-dose corticosteroid (prednisolone, ≤5 mg/day). A biological DMARD, infliximab, had been used in one (6%) patient. Nine (50%) patients were treatment-naïve for RA. Table 2 shows the study treatments administered for RA-ILD. The indications for RA-ILD therapy were progressive RA-ILD (N = 15; 83%) and AE-RA-ILD (N = 3; 17%). All patients received prednisolone, administered as monotherapy (N = 11; 61%), combination therapy with tacrolimus (N = 2; 11%), monotherapy (25–50 mg/day) after methylprednisolone pulse therapy (N = 4; 22%), or combination therapy at a dose of 40 mg/day with tacrolimus following methylprednisolone pulse therapy (N = 1; 6%). In 13 patients treated with prednisolone alone or prednisolone and tacrolimus without steroid pulse therapy, the initial dose of prednisolone was <30 mg/day in one patient, 30–40 mg/day in 10 patients, and >40 mg/day in two patients.
Table 1.Baseline patient demographic and disease characteristics
| Characteristic | N = 18 |
|---|---|
| Age, years | 72 (49–85) |
| Sex | |
| Male | 9 (50) |
| Female | 9 (50) |
| mMRC dyspnea scale score | |
| 0 | 3 (17) |
| 1 | 9 (50) |
| 2 | 2 (11) |
| 3 | 3 (17) |
| 4 | 1 (6) |
| Smoking status | |
| Never | 9 (50) |
| Current or former | 9 (50) |
| Years since onset of RA | 1 (0–18) |
| PaO2 on room air (Torr)a | 72 (61–98) |
| LDH, U/mL | 218 (158–370) |
| KL-6, U/mL | 990 (390–2909) |
| SP-D, ng/mL | 152 (30–325) |
| FVC, L | 2.13 (0.6–3.79) |
| FVC, % of predicted | 80 (30–116) |
| DLCO, mL/min per kPaa | 9.9 (4.1–15.1) |
| DLCO, % of predicteda | 54 (21–104) |
| 6-minute walk distance, ma | 345 (185–580) |
| HRCT pattern | |
| UIP | 1 (6) |
| Probable UIP | 1 (6) |
| Indeterminate for UIP | 0 (0) |
| Alternative diagnosis of UIP | 16 (88) |
| NSIP pattern | 8 (44) |
| OP pattern | 2 (11) |
| NSIP + OP pattern | 6 (33) |
| Baseline treatment for RA | |
| None | 9 (50) |
| Synthetic DMARDs | 9 (50) |
| Methotrexate | 1 (6) |
| Salazosulfapyridine | 5 (28) |
| Bucillamine | 1 (6) |
| Tacrolimus | 2 (11) |
| Iguratimod | 1 (6) |
| Synthetic DMARDs + low-dose prednisolone (≤5 mg/day) | 3 (17) |
| Biological DMARDs | 1 (6) |
| Infliximab | 1 (6) |
Data are shown as median (range) or N (%). a Data were not available in two patients. DLCO, diffusing capacity of lung carbon monoxide; DMARDs, disease-modifying anti-rheumatic drugs; FVC, forced vital capacity; HRCT, high-resolution computed tomography; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; mMRC, modified Medical Research Council; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; PaO2, arterial oxygen pressure; RA, rheumatoid arthritis; SP-D, surfactant protein-D; UIP, usual interstitial pneumonia
Table 2.Treatments administered for RA-ILD
| Agents and initial doses of prednisolone | N = 18 |
|---|---|
| Prednisolone alone | 11 (61) |
| >40 mg/day | 2 (11) |
| 30–40 mg/day | 8 (44) |
| < 30 mg/day | 1 (6) |
| Methylprednisolone pulse + prednisolone | 4 (22) |
| >40 mg/day | 1 (6) |
| 30–40 mg/day | 2 (11) |
| < 30 mg/day | 1 (6) |
| Prednisolone + tacrolimus | 2 (11) |
| 30–40 mg/day | 2 (11) |
| Methylprednisolone pulse + prednisolone + tacrolimus | 1 (6) |
| 30–40 mg/day | 1 (6) |
Data are shown as N (%).
ILD, interstitial lung disease; RA, rheumatoid arthritis.
Efficacy
A database lock was performed on March 28, 2022. The median follow-up in all patients was 12 months (range, 3–12), and there were two deaths from AE-RA-ILD or prostate cancer. Among patients alive at month 12, cumulative doses of prednisolone during 12-month treatment were <10 g in 7 patients, 10–12 g in 4 patients, and 12 g in 5 patients. For the primary endpoint, information on FVC at month 12 was unavailable in four (22%) patients because of death (N = 2; 11%), protocol violations (N = 1; 6%), or poor general condition (N = 1; 6%). FVC at month 12 was improved or stabilized in six patients each, resulting in an improvement or stabilization rate of 67% (95% confidence interval [CI], 41%–87%; Figure 1a). The mean absolute change in FVC from baseline was +240 mL, but did not reach statistical significance (P = 0.11; Figure 1b). The proportions of patients with improved or stabilized FVC were 67% (95% CI, 41%–87%) at month 3 (Figure 1c) and 50% (95% CI, 26%–74%) at month 6 (Figure 1d). For DLCO, data were unavailable for six (33%) patients at months 3, 6, and 12. The proportions of patients with improved or stabilized DLCO were 50% (95% CI, 26%–74%) at month 3, 44% (95% CI, 22%–69%) at month 6, and 56% (95% CI, 31%–79%) at month 12. Regarding severity of dyspnea, the mMRC scores had improved by at least one grade in seven (39%) patients at month 3, six (33%) patients at month 6, and seven (39%) patients at month 12 (Figure 1e). Compared with baseline, the HRCT findings had improved or stabilized in 13 (72%) patients at month 3, 14 (78%) patients at month 6, and 15 (83%) patients at month 12 (Figure 1f). Representative HRCT images showing improvement, stabilization, or progression at 3 months after treatment initiation are presented in Figure 2.

Figure 1:Efficacy of treatments for RA-ILD. a) Proportions of patients with improved or stabilized FVC and declined FVC at month 12. b) Mean changes in FVC from baseline to month 12. The bars indicate the standard error. The Wilcoxon rank-sum test was used. c) Proportions of patients with improved or stabilized FVC and declined FVC at month 3. d) Proportions of patients with improved or stabilized FVC and declined FVC at month 6. e) Changes in mMRC dyspnea scores from baseline to month 12. The Wilcoxon rank-sum test was used. f) Proportions of patients with improved or stabilized HRCT findings and progressed HRCT findings from baseline at months 3, 6, and 12. FVC, forced vital capacity; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; mMRC, modified Medical Research Council; RA, rheumatoid arthritis.

Figure 2:Representative axial HRCT images at baseline and month 3. a) HRCT images in Case 7 showing improvement of consolidation and ground-glass opacity in response to treatment. b) HRCT images in Case 10 categorized as stabilized after treatment. c) HRCT images of findings refractory to treatment in Case 2 showing progression of reticular shadow and fibrosis. HRCT, high-resolution computed tomography.
To provide clinically relevant insights using our observations, we created a composite classification of patient outcomes based on assessment of the changes in mMRC score, FVC, and HRCT findings. Patients were categorized as improved when ≥2 of the three factors showed improvement and as progressed when ≥1 of the three factors showed progression. Patients lacking evaluation of ≥2 of the three factors were considered undeterminable. Other patients were considered stabilized (Figure 3a). Using this composite classification, seven (39%), four (22%), and six (33%) patients were categorized as improved at months 3, 6, and 12, respectively (Figure 3b). Furthermore, three (17%), five (28%), and five (28%) patients were categorized as stabilized at months 3, 6, and 12 (Figure 3b).
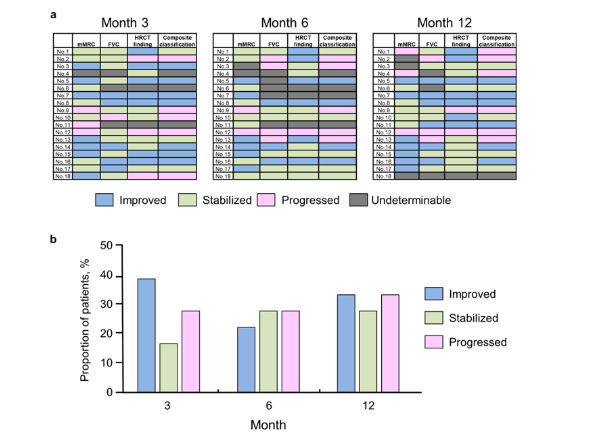
Figure 3:Composite classification of patient outcomes based on changes in mMRC score, FVC, and HRCT findings. a) Heat map representation of the changes in mMRC score, FVC, and HRCT findings, along with the composite classification at months 3, 6, and 12. Each field is color-coded in blue (improved), green (stabilized), pink (progressed), or gray (undeterminable). b) Proportions of the outcomes according to the composite classification at months 3, 6, and 12. FVC, forced vital capacity; HRCT, high-resolution computed tomography; mMRC, modified Medical Research Council.
Adverse events
A total of eight treatment-related adverse events were documented in five (28%) patients. Worsened blood sugar control in patients with preexisting type 2 diabetes mellitus was the most frequent adverse event (N = 4; 22%), followed by cytomegalovirus infection (N = 1; 6%), elevated transaminases due to concomitant use of trimethoprim-sulfamethoxazole (N = 1; 6%), depression (N = 1; 6%), and osteonecrosis of the femoral head (N = 1; 6%). There were no life-threatening adverse events or treatment-related deaths.
Discussion
The observations in this multicenter, prospective, clinical study suggest that anti-inflammatory therapeutics, namely corticosteroids, are effective and feasible for treatment of patients with RA-ILD requiring medical intervention. In the study, indications for treatments and therapeutic agents for RA-ILD were not prespecified and were at the discretion of the attending pulmonologists. Furthermore, the study was conducted before nintedanib was approved for PF-ILD. On HRCT, the majority of patients had an NSIP pattern, and only two patients had a UIP or probable UIP pattern. Therefore, based on the clinical decisions made by individual physicians, our cohort appeared to be favorably selected for anti-inflammatory therapeutics, leading to the use of corticosteroids in all patients as key drugs. Nevertheless, to our knowledge, this is the first study to prospectively assess the treatment landscape for RA-ILD and the efficacy and safety of treatments in a real-world setting.
In contrast to IPF, for which immunosuppressive therapy is associated with worse outcomes and safety concerns [17], it remains unclear whether immunosuppressive therapy is beneficial for patients with RA-ILD. Traditionally, immunosuppressive agents have generally been used as therapeutics for RA-ILD regardless of fibrosis [5]. In patients with RA-UIP, a retrospective study found that treatment with corticosteroids with or without other immunosuppressive medications improved or stabilized the disease in approximately 50% of 84 patients without a significant survival benefit [15]. In another retrospective study on 40 patients with RA-ILD, in which an indeterminate pattern with diffuse ground-glass opacity and reticulation was the most prevalent pattern on HRCT, prednisone therapy with an initial high-dose (1 mg/kg/day) scheme for 6 weeks followed by a reduction scheme ending with a dose of 10 mg/day at 6–8 months led to improvements in FVC [18]. In a more recent retrospective case series of 26 patients with CTD-ILDs, including 11 RA-ILD cases, pulse dose methylprednisolone therapy followed by 1 year of tacrolimus combination therapy with corticosteroids resulted in multidimensional improvement, with 11 of the 26 patients having a UIP pattern on pathological or radiological review [19]. In the present study, the mean changes in FVC from baseline at months 3, 6, and 12 were +199, +73, and +240 mL, respectively, demonstrating relatively long-lasting efficacy. Using our composite classification, 33% and 28% of patients were categorized as improved and stabilized at month 12, respectively. However, the interpretation of these results warrants caution because the study had no control arm and the natural history of RA-ILD, particularly inflammatory-phenotype RA-ILD, remains largely unknown. In a study using data from the placebo group in the INBUILD trial [8], which involved patients with PF-ILDs other than IPF, including RA-ILD, the proportion of patients with a relative decline in FVC >10% predicted at week 52 was 48.9%, with similar annual rates of decline in FVC across five prespecified ILD groups (hypersensitivity pneumonitis; autoimmune ILDs; idiopathic NSIP; unclassifiable idiopathic interstitial pneumonia; other ILDs) [20]. These findings suggest that as many as 50% of patients with PF-ILDs other than IPF who receive appropriate management, except for nintedanib, may achieve a stabilized disease course in accordance with our definition. Collectively, the present findings warrant further validation in a larger prospective cohort of patients or even a randomized controlled trial to explore the efficacy of anti-inflammatory therapy in patients with RA-ILD, particularly the effectiveness according to the disease phenotypes to establish tailored therapies.
Feasibility is a major concern for anti-inflammatory therapy in patients with RA. In the present study, corticosteroid-centered therapies were largely tolerated without any life-threatening adverse events or treatment-related deaths. However, this may have been underestimated because of the small sample size. Considering that patients with RA-ILD who received daily prednisone doses above 10 mg/day were reported to be at high risk of serious infection requiring antimicrobial therapy and hospitalization [21], safety and an appropriate tapering strategy for corticosteroids should be determined in future studies.
The present study had several limitations. First, despite the prospective design of the study, the baseline conditions of the disease were heterogeneous and three cases of AE-RA-ILD were included. In addition, the therapeutic strategy was not prespecified, and thus a variety of treatments were prescribed. Second, some patients had unavailable data for longitudinal assessment of variables. Finally, the study was unable to address the impact of the treatments for RA-ILD on survival outcomes owing to the very limited number of deaths. These limitations preclude the drawing of definitive conclusions from the study data.
Conclusion
The present study, conducted in a real-world setting before the approval of antifibrotic therapy, has demonstrated that corticosteroids play a major role in the treatment of RA-ILD and offer disease improvement or stabilization in terms of FVC and composite outcomes in approximately 60% of patients at month 12 after treatment initiation. While corticosteroids have been considered a mainstay for clinical management of CTD-ILDs, including RA-ILD, despite a lack of robust data to guide their use [4,22], the present study underscores the importance of corticosteroid-centered therapeutics, at least for the subset of patients with progressive RA-ILD. Larger prospective trials are warranted to confirm the present findings and thus facilitate the development of personalized therapy for RA-ILD tailored to the disease phenotype and severity to improve the outcome of individual patients.
Abbreviations:
AE: acute exacerbation; CTD: connective tissue disease; DLCO: diffusing capacity of lung carbon monoxide; DMARDs: disease-modifying anti-rheumatic drugs; FVC: forced vital capacity; HRCT: high-resolution computed tomography; ILD: interstitial lung disease; KL-6: Krebs von den Lungen-6; LDH: lactate dehydrogenase; mMRC: modified Medical Research Council; NSIP: nonspecific interstitial pneumonia; OP: organizing pneumonia; PaO2: arterial oxygen pressure; PF: progressive fibrosing; RA: rheumatoid arthritis; SP-D: surfactant protein-D; STROBE: Strengthening the Reporting of
Observational Studies in Epidemiology; UIP: usual interstitial pneumonia.
Trial registration number: UMIN000020208
Date of registration: 2016/1/12
Acknowledgments
The authors would like to thank the patients, their families, and all the investigators who participated in the study. The authors also thank Alison Sherwin, PhD, from Edanz (htpps://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contributions
Mineo Katsumata: Conceptualization, Acquisition of data, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – Original Draft, Writing – Review & Editing.
Yusuke Inoue: Conceptualization, Acquisition of data, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – Original Draft, Writing – Review & Editing.
Shiro Imokawa: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Naoki Koshimizu: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Toshihiro Shirai: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Mikio Toyoshima: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Hideki Yasui: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Masato Karayama: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Yuzo Suzuki: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Hironao Hozumi: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Kazuki Furuhashi: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Noriyuki Enomoto: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Tomoyuki Fujisawa: Acquisition of data, Data curation, Supervision, Validation, Writing – Review & Editing.
Naoki Inui: Acquisition of data, Data curation, Project administration, Supervision, Validation, Writing – Review & Editing.
Takafumi Suda: Acquisition of data, Data curation, Project administration, Supervision, Validation, Writing – Review & Editing.
Funding
None.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. N Engl J Med. 2020;383(10):958-68.
2. Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018;320(13):1360-72.
3. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863-77.
4. Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63 Suppl 5:v1-58.
5. Kadura S, Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev. 2021;30(160):210011.
6. Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford). 2007;46(2):350-7.
7.Yamakawa H, Ogura T, Kameda H, Kishaba T, Iwasawa T, Takemura T, et al. Decision-Making Strategy for the Treatment of Rheumatoid Arthritis-Associated Interstitial Lung Disease (RA-ILD). J Clin Med. 2021;10(17):3806.
8. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381(18):1718-27.
9. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-81.
10. Maher TM, Tudor VA, Saunders P, Gibbons MA, Fletcher SV, Denton CP, et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med. 2023;11(1):45-54.
11. Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(5):543-8.
12. Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47.
13. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194(3):265-75.
14. Hozumi H, Kono M, Hasegawa H, Kato S, Inoue Y, Suzuki Y, et al. Acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: mortality and its prediction model. Respir Res. 2022;23(1):57.
15. Song JW, Lee HK, Lee CK, Chae EJ, Jang SJ, Colby TV, et al. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(2):103-12.
16. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-8.
17. Idiopathic Pulmonary Fibrosis Clinical Research N, Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr., Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093-101.
18. Rojas-Serrano J, Gonzalez-Velasquez E, Mejia M, Sanchez-Rodriguez A, Carrillo G. Interstitial lung disease related to rheumatoid arthritis: evolution after treatment. Reumatol Clin. 2012;8(2):68-71.
19. Yamano Y, Taniguchi H, Kondoh Y, Ando M, Kataoka K, Furukawa T, et al. Multidimensional improvement in connective tissue disease-associated interstitial lung disease: Two courses of pulse dose methylprednisolone followed by low-dose prednisone and tacrolimus. Respirology. 2018;23(11):1041-8.
20. Brown KK, Martinez FJ, Walsh SLF, Thannickal VJ, Prasse A, Schlenker-Herceg R, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J. 2020;55(6):2000085.
21. Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Risk of serious infection in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2016;35(10):2585-9.
22. Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016;352:h6819.
Received:April 17, 2024;
Accepted: May 2, 2024;
Published: May 6, 2024.
To cite this article : Katsumata M, Inoue Y, Imokawa S, Koshimizu N, Shirai T, Toyoshima M, et al. Effects of Physical Therapy Intervention in Pneumonia Patients According to the Severity of Illness. European Journal of Respiratory Medicine. 2024; 6(1): 403 - 408. doi: 10.31488/ EJRM.143.
© The Author(s) 2024. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
