Mini Review/ Open Access
DOI:2020; 2(1): 143 - 149 . doi: 10.31488/ejrm.109
Lung Cancer and COVID-19: Therapeutic Implications
Raquel Laza-Briviesca,Virginia Calvo,Alberto Cruz-Bermúdez, Fernando Franco, Mariano Provencio*
Medical Oncology Department, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, Spain
*Corresponding author: Prof. Mariano Provencio, Medical Oncology Department, Puerta de Hierro Hospital.C/Manuel de Falla, 28222, Majadahonda,Madrid, Spain.
Abstract
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected millions of people and has already resulted in hundreds of thousands of deaths, affecting more than 200 countries. The medical crisis situation experienced has forced clinical decisions that are not always supported by hard data. This is even more dangerous in the situation of lung cancer patients, since, lungs are the tissue predominately affected by SARS-CoV-2, these patients are potentially more fragile, and are under different oncological treatments.In this mini-review we examine the most relevant studies currently available, to manage lung cancer patients in the best possible way, reviewing relative risks of patients and specific problems of differential diagnosis among COVID-19 and lung cancer, in ordertoadapttreatment strategies for these patients. Overall, larger cohorts of lung cancer patients and specific immune system reports need to be carried out to improve patients care.
Keywords: Lung cancer, COVID-19, SARS-CoV-2
Introduction
COVID-19 outbreak and riskfor lung cancer patientsSevere acute respiratory syndrome coronavirus (SARS-CoV-2) emerged in Wuhan, China, in December 2019 [1]. The resulting illness, COVID-19, has led to an unprecedented pandemic situation, all over the world, with thousands of deaths reported, involving more than 200 countries. COVID-19 is often associated to invasive mechanical ventilation, extrapulmonary organ dysfunction and substantial in-hospital mortality [2].
Epidemiological data indicates that a huge variability between COVID-19 patients,with 81% of patients with mild or moderate symptoms, 14% with severe disease and 5% with critical condition requiring admission to intensive care unit (ICU). Higher morbidity and mortality risk has been described in patients older than 65 years old, hypertension, cardiovascular disease, diabetes, chronic respiratory disease, renal disease, obesity and cancer [3–10].
Moreover, patients with cancer may have an additional risk of infection with SARS-CoV-2 due to their cancer treatments, such as, chemotherapy, immunotherapy, radiation or surgery, which are myelosuppressants [11], or immunomodulatory drugs [12]and therefore likely to develop a severe event and died of COVID-19 [13]. However, other studies have shown no association between chemotherapy [10] or immunotherapy[14,15] with increased risk of severity of COVID-19.
As COVID-19 pandemic develops, more studies focused on cancer are published. In Liang study on a Chinese cohort, when comparing cancer patients with the rest of the cohort, cancer patients were older (mean age 63.1 years old vs 48.7), had higher smoking history (22% vs 7%) and had higher basal infiltration in the computed tomography (CT), however no differences were found in sex, baseline symptoms, comorbidities or baseline severity of x-ray. Moreover, patients with cancer had higher risk of developing severe events, including ICU admission and ventilation requirement (39% vs 8%) [16]. Liang et al., also describe a higher incidence than expected (0.29%) of patients with cancer history, with 18 (1%, CI: 0.61-1.61) out of 1590 cases with COVID-19, 5 of them (28%) had lung cancer.
On the other side, in a study in Italy, with a similar median age of 63 year old,hypertension and cardiovascular diseases as the most common comorbidities, 4% of patients had chronic obstructive pulmonary disease and8% of patients who were admitted to the ICU had an active or prior malignancy [17]. Moreover, in a study in New York, with a median age of 65 years old, hypertension, diabetes and obesity as the most often comorbidities, 6% of patients hospitalized had an active cancer [9].Similar results were found in a larger cohort in Spain with preliminary results indicating prevalence of 8.5% [18].
It seems that an irregular consolidation pattern in the CT and treatment received in the last 14 days of their ICU admission were associated with higher risk of severe events, it was found in 53.6% of patients [19], including admission to ICU,invasive ventilation requirement or death [16]. Additionally, severe COVID-19 clinical syndrome, which is developed in a subset of patients and is characterized by respiratory failure or death has been attributed to a cytokine storm [20].
The analysis of two non-diagnosed COVID-19 cases at the time of lung cancer surgery, showed edema, protein exudate, focal reactive hyperplasia of pneumocytes with patchy inflammatory cellular infiltration, and multinucleated giant cells [21] which is similar to histological examinations on COVID-19 positive patients without cancer [22]. Although the initial phase of the infection in which these cancer patients were studied may evolve during COVID-19 development.
Even though it is difficult to have any conclusion, other studies had support the lack of information and the vulnerability of cancer patients in China [23], as well as in Europe [24] including age, gender and comorbidities as COVID-19 related deaths. However, larger studies on cancer patients and SARS-CoV-2 infected patients, have demonstrated that cancer types, cancer stage and cancer treatment contribute to severe events development [25,26] as well as tumor status (active, stable or progressing) with cancer patients prognosis with COVID-19 [27]. In our Hospital we have register patients with lung cancer and COVID-19 with approximately 25% of mortality, similar to Memorial Sloan Kettering Cancer CenterGroup [28] but inferior to TERAVOLT study [13].These results seem to indicate that the mechanism in cancer patients is similar to that observed in the general population.
Diagnosis of COVID-19 in lung cancer patients.
It has been described that many COVID-19 patients are asymptomatic, however, when symptoms are present these are predominantly respiratory symptoms, being the most frequent symptom cough (86%) being or not associated to fever[22]. From patients with SARS-CoV-2 pneumonia, 7% are adult patients critically ill and 5% had respiratory distress that required ICU admission [29].
We could suspect presence of COVID-19 disease if leukopenia or lymphopenia are present, as well as elevated transaminases, C-reactive protein, d-dimer, ferritin and lactate dehydrogenase [30]. Serological biomarkers associated to SARS-CoV-2 infection are describe in Table 1 [31]. Additionally an increase in interleukin 6 can be observed during the cytokine storm, that could be indicator of systemic disease, including renal or cardiogenic failure [32].
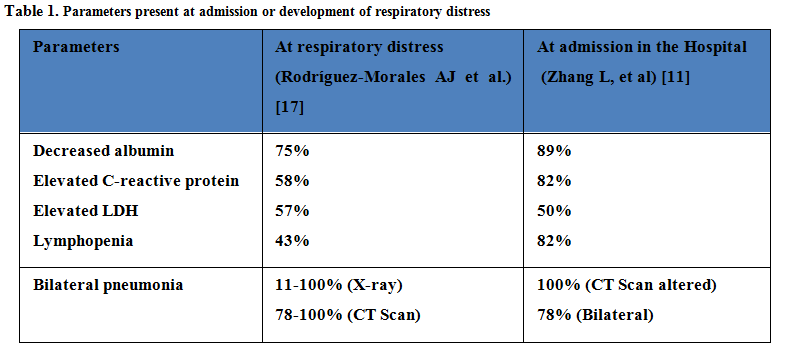
Remarkably, many of these symptoms are present in lung cancer patients [33], therefore distinguish between lung cancer and COVID-19 could be problematic, even more in patients without fever. Other symptoms that do not normally appear in lung cancer patients could be helpful, as diarrhea (27%) or other digestive aspects, headache, anosmia or ageusia, among others [34].To further complicate the diagnosis, these alterations might not be present in early COVID-19 patients and they might be present in lung cancer patients due to the tumor or to the treatment that they are receiving [35]. Indeed, the radiological features associated to SARS-CoV-2 infection are similar to toxicities caused byimmunotherapy treatmentsin lung cancer patients, increasing the struggleof COVID-19 diagnosis in these patients [36].
Nevertheless, it seems that bilateral ground-glass opacities (GGO) on CT might be representative of COVID-19, being present between 57% [37]and 98% [38]of patients.
Patients could also develop consolidation patterns, with or without previous GGO implying a worst progression of the disease [39,40]. In any case, GGO by itself or consolidation patterns happened to be associated with peripheral distribution or bilateral involvement41, but more studies are reporting other characteristics [42,43], for example, a reticular pattern of interstitial lymphocytic infiltration with many ground-glass opacities [44] or others patterns like crazy paving [45]. COVID-19 infection can cause pleural alterations, including lymphadenopathy o pericardial effusion [46], also found in advanced lung cancer patients.
Some additional complications could appear when other diseases manifest similar patterns, such as infection by Pneumocystis jiroveci, Mycoplasma or others viral diseases, non-invasive lung adenocarcinoma, carcinomatous lymphangitis, pulmonary alveolar proteinosis, sarcoidosis or post-radiotherapy pneumonitis.
The World Health Organization (WHO) has recommended to take a nasopharyngeal swab or a sputum using the appropriated packing and shipment and test it using PCR technique [47]. In addition, importance of knowing the viral load has been demonstrated, since severe cases present 60 times more viral load than mild cases[48].
To summarize, diagnosis of COVID-19 is crucial and false negatives can occur if the infection is recent or if the specimen is not collected properly. Therefore, strategies to improve early detection and reduce infections are priorities for oncologic care.
Treatment related adverse effects influence on COVID-19 diagnosis.
Specific lung cancer treatment related adverse events (TRAEs) are compatible with COVID-19, such as those derived from the use of ALK-tyrosine kinase inhibitors (TKIs), EGFR-TKIs, or Immunotherapy.
The incidence of pulmonary toxicity in lung cancer patients by ALK-TKIs varies from 2 to 6%, although there are publications that suggest a higher incidence on Japan origin population compared to non-Japan origin population, with mortality rates of 0-19% [49]. Accordingly, the incidence of Interstitial lung disease (ILD) in Asian patients from the J-ALEX study was 8%, while in non-Asian patients from the ALEX study ILD was 1% [50,51].
The pattern of pulmonary involvement, secondary to ALK-TKIs, is usually organized pneumonia with generally good response to corticosteroids [52]. The timing of toxicity presentation does not help in the differential diagnosis of COVID-19, as it can occur from a few days to several months after treatment initiation, with the exception of Brigatinib, for which some studies have reported few cases of early toxicity within the first 7 days of treatment [51–53]. ILD is more frequent in smokers and has been associated to renal function reduction, older age, and previous pleural effusion [54].
The overall incidence of pneumonitis in patients treated with EGFR-TKI is 1.12%, this incidence is again higher in the Japanese population [55]. Similar differential diagnosis should be considered when using Osimertinib, in which an ILD incidence of 1-2.37% has been reported [56,57]. It should be noted that many drugs studied in the early stages can also develop ILD, even before developing obvious symptoms[58].
Additionally, about 3-6% of patients receiving immunotherapy during SARS-CoV-2 infection, may develop immune-related pneumonitis, which raises the question of differential diagnosis of COVID-19 [59].
COVID-19 treatment for lung cancer patients.
To date, there is no treatment approved by FDA or EMA for COVID-19 patients since none of them have clearly demonstrated to be effective and safe. Many clinical trials are being developed to solve this issue, but severe adverse eventsare a drawback and the best option for cancer patients is still not clear. Occasionally, the lack of suitable treatment for these patients is leading us to provide different drugs, without the appropriate safety and efficacy required for clinical practice.
To improve treatment on cancer patients, medical oncology societies have developed clinical guidelines to minimize the negative impact of the pandemic, classifying patients in three groups by their cancer treatment priority (progressive tumors, stable tumors and palliative care) [60–62], mostly based on common sense rather than objective information63. However, more data is needed to give our patients the best care.
Clinical guidelines are very much needed, but as an example of the risk that could entails, one of the recommendation is the use of prophylactic granulocyte colony-stimulating factor 1 (G-CSF-1) [63] when, for example Pembrolizumab plus chemotherapy has around 5% of febrile neutropenia. We kindlydisagree with this suggestion, since the severity of SARS-CoV-2 infection seems to be more correlated with a cytokine release storm than with conventional sepsis or infection64 and it could be more harmful than beneficial for patients as we do not have enough evidence to support it.
As a matter of fact, there are many case reports of cancer patients treated with G-CSF-1in a context of neutropenic infection [65,66], where their severity is more associated to ahyper inflammatory syndrome than with a usual sepsis situation, and therefore G-CSF-1 for SARS-CoV-2 may not be useful and even harmful for patients [67,68].
COVID-19 pandemic is an extreme situation where we all need to practice medicine under pressure and in stressful ways, being not the best conditions to treat our patients with unsafe treatments without enough evidence.
We should be aware of the limited information that we have to generate clinical guidelines, that could cause severe damage to the health system, to offer the best health care to the patients, we should take into account the demographic characteristics of the population, where they live, the incidence of SARS-CoV-2 infection and the infrastructure of the health system that they can access.
Treatment strategy adapted to COVID-19 pandemic.
Medical oncologists should take special considerations to know the situation of each patient, their specific risk circumstances, and ameliorate their SARS-CoV-2 infection, to the extent possible without affecting their cancer care.
With few studies including lung cancer and COVID-19 patients, seems wise to adapt and modify each situation not only depending on the patient circumstances, but also regionally, depending on pandemic context [63]. At the clinical practice, we have changed administration of drugs extending their lapse time but it is crucial to differentiate between being at the peak of the incidence curve, with excessive affected patients and difficulties in performing quality medical attention, and other gentlerpandemic scenarios. Lung cancer targeted treatment could be maintained due to their efficacy and in the case of starting a new treatment, the ones with lower risk of ILD should be selected.
However, there are some common considerations in the management of cancer patients that can be made, such as; temperature testing, preparing patients that need to be admitted in ICU, limiting the access to relatives for visiting, creating a special organization to track potential infected patients, establishing a security distance between patients and medical oncologist or including online consultation to avoid exposure to infection among others [69].
Additionally, in thisexceptional situation where patients are developing an aggressive systemic disease, is essential to treat patients as soon as possible, use the UCI appropriately and test for possible pathogens associated.Importantly, these measures should be included in routine practice together with diagnostic test for SARS-CoV-2 infection and serological test for immune status, due to their importance, and more so in patients with cancer considering their vulnerability condition [25].
Whether COVID-19 diagnosis is confirmed, medical oncologist should consider benefits and risk of their cancer patients having in consideration many factors, such as infection incidence, clinical characteristics of patients, local population and health system structures, if hospitals are well equipped with not many COVID-19 patients, oncologic treatment should continue, otherwise if resources are limited, only oncologic emergencies with higher risk of mortality and morbidity should be treated.
Surgeries on lung cancer patients with COVID-19
It has been described that asymptomatic patients, who underwent electivesurgery during the SARS-CoV-2 incubation period, developed COVID-19 symptoms after surgery and later on, theywere diagnosed with COVID-19 by PCR. These patients received various surgical treatments, catalogued according to the level of complexity, with 58.8% of patients being level 3 with moderate risk and technical difficulty, including thoracoscopies with lobectomy. Fifteen of 34 (44%) patients required ICU admission, a proportion significantly higher than patients with SARS-CoV-2 infection and no surgery, which is usually around 26%.
This information suggests that surgical procedures can accelerate and exacerbate COVID-19 progression. Regarding mortality, 20.6% of patients, who underwent surgery, died, a much higher rate than expected in this type of surgeries (all of them level 3, such as lobectomies), which is usually situated around 2% [70].
Nevertheless, another feature of interest are the implications of delaying elective surgeries in the treatment of cancer. According to a recently published estimation model [71], a 6-month surgery delay, would cause a reduction of 27% in survival rate for stage I, 33.7% for stage II and 28.9% for stage III patients, for lung cancer patients between 60-69 years old.For all these reasons, it seems quite advisable and prudent to establish a prior diagnosis of COVID-19 and in case of positivity, establish a period of quarantine before proceeding with an elective surgery of this type.
Life after the pandemic's peak.
The circumstances described here make it necessary to take medium to long-term actions after the initial pandemic peak.Patients` infection status must be available at the beginning of the oncological treatment or its resumption, either by serological, PCR, or both tests, depending on the results obtained.
Neutralizing antibodies play an important role at different levels, from direct protection against viral infection and the disease evolution in the patient, to determine their current infection status, and even for the development of treatments based on recovered- patients plasma [21], as well as, for testing effectiveness of new vaccines [72]. Therefore, learning about how SARS-CoV-2 neutralizing antibodies evolve during the course of COVID-19 disease in this pandemic is crucial (Table 2).
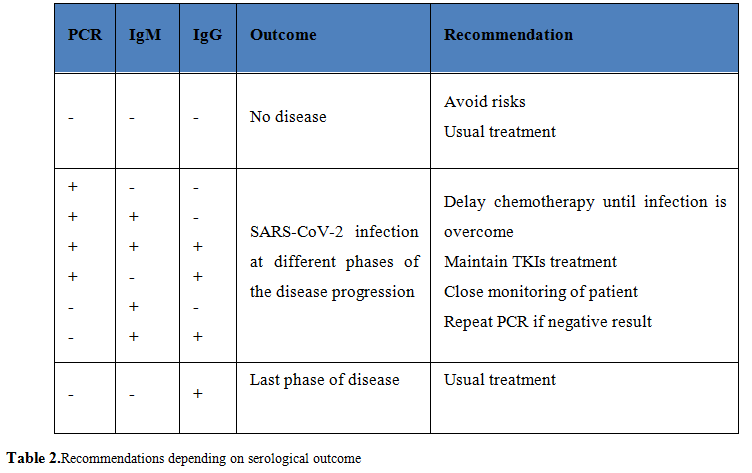
The average seroconversion time of IgG and IgM detected by ELISA is about 17-20 days, respectively, up to 30 days after infection [73], with possible variations in the two-phase course of the IgG response and potentially fluctuations due to the use of corticosteroids [74].However, once again, we have very little evidence. There are studies that associate higher antibody titers to older ages. It is estimated that 30% of patients will not develop detectable antibody titers after infection with SARS-CoV-2 [75].
It remains to be investigated what risk of re-infection these patients have. In patients where IgG after infection is not conclusive, other laboratory test involving immune mechanisms could be done, such as cytokines measurement or characterization of the immune cell response [76].In any case, what is recommended is to carry out tests with a high percentage of sensitivity and contrasted specificity [77].
Conclusion
To conclude, in this pandemic situation, a collapsed healthcare system is causing an impact on diagnosis, treatment and mortality of oncologic patients. As we have mention along the manuscript, balance between benefits and risks for cancer patients is crucial. Therefore, medical oncologist are the ones that should decide if cancer patients during COVID-19 pandemic will benefit from oncologic treatments, their morbidity and mortality risks caused by SARS-CoV-2and also consider when telehealth could be of useful during follow-up in order to limit possible infections during hospitals visits and to protect patients with cancer affected by COVID-19 pandemic.
Acknowledgments
R.L-B, was supported by PEJ16/MED/AI-1972 and PEJD-2018-PRE/SAL-8641 from European Social Fund and Comunidad de Madrid, both granted to M.P. A.C-B, is supported by a ISCIII- “Sara Borrell” contract CD19/00170.
Conflict of interests
R.L-B., A.C-B and F.F. declare no conflict of interest.M.P. reports grants, personal fees and non-financial support from BMS, grants, personal fees and non-financial support from ROCHE, grants, personal fees and non-financial support from ASTRAZENECA, personal fees from MSD, personal fees from TAKEDA, outside the submitted work.V.C. reports personal fees from Roche, BMS, MSD, AstraZeneca, Boehringer.
Abbreviations
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; ICU: intensive care unit; CT: computed tomography; GGO: ground-glass opacities; WHO; World Health Organization; TRAEs: treatment related adverse events; TKIs: tyrosine kinase inhibitors; ILD: Interstitial lung disease; G-CSF-1: granulocyte colony-stimulating factor [1].
References
1. Raoult D, Zumla A, Locatelli F, et al. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4(4):66-75.
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770.
3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
4. Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020: NEJMoa2002032.
5. Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. April 2020. doi:10.1007/s15010-020-01432-5
6. People Who Are at Higher Risk for Severe Illness | CDC.
7. Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
8. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. JAMA. April 2020.
9.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. April 2020.
10. Lee LYW, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet. 2020;395(10241):1919-1926.
11. Patel R, Park J, Shah A, Saif MW. COVID-19 and Cancer Patients. Cancer Med J. 2020;3(1):40-48.
12. Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107-113.
13. Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914-922.
14.Luo J, Rizvi H, Egger J V., Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. Cancer Discov. 2020;10(8):1121-1128.
15. Gonzalez-Cao M, Antonazas-Basa M, Puertolas T, et al. Cancer immunotherapy does not increase the risk of death by COVID-19 in melanoma patients. medRxiv. May 2020:2020.05.19.20106971.
16. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337.
17. Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA - J Am Med Assoc. 2020;323(16):1574-1581.
18.Fabio Franco F, Cobo M, Rodriguez-Abreu D, et al. Seroprevalencia de SARS-CoV2 entre pacientes con cáncer de pulmón en España. Estudio SOLID del Grupo español de Cáncer de Pulmón (GECP). RESULTADOS PRELIMINARES. In: SEOM2020.
19.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;0(0).
20. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53(1):19-25.
21. Tian S, Hu W, Niu L, et al. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. J Thorac Oncol. 2020;15(5):700-704.
22. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;2600(20):19-21.
23. Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 Transmission in Patients with Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020.
24. Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA - J Am Med Assoc. 2020.
25. Dai M-Y, Liu D, Liu M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multi-Center Study During the COVID-19 Outbreak. SSRN Electron J. 2020.
26. Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309-1316.
27. Albiges L, Foulon S, Bayle A, et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat Cancer. 2020;1(October).
28. Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31(10):1386-1396.
29. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020.
30. Zheng C, Wang J, Guo H, et al. Risk-adapted Treatment Strategy For COVID-19 Patients. Int J Infect Dis. 2020;94:74-77.
31. Rodriguez-Morales AJ, Cardona-Ospina JA. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020.
32. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513.
33. Provencio M, Carcereny E, Rodríguez-abreu D, et al. Lung cancer in Spain: information from the Thoracic Tumors Registry ( TTR study ). Transl Lung Cancer Res. 2019;8(4):461-475.
34. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020.
35. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918.
36. Kumar S, Chmura S, Robinson C, et al. Alternative Multidisciplinary Management Options for Locally Advanced Non-Small Cell Lung Cancer During the COVID-19 Global Pandemic. J Thorac Oncol. 2020.
37. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-NCoV). Radiology. 2020;295(1):202-207.
38. Li K, Wu J, Wu F, et al. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Invest Radiol. February 2020.
39. Wu J, Wu X, Zeng W, et al. Chest CT Findings in Patients with Coronavirus Disease 2019 and Its Relationship With Clinical Features. Invest Radiol. 2020;55(5):257-261.
40. Song F, Shi N, Shan F, et al. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295(1):210-217.
41. Zhao W, Zhong Z, Xie X, et al. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. Am J Roentgenol. 2020;214(5):1072-1077.
42. Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothorac Imaging. 2020;2(2): e200152.
43. Qian L, Yu J, Shi H. Severe Acute Respiratory Disease in a Huanan Seafood Market Worker: Images of an Early Casualty. Radiol Cardiothorac Imaging. 2020;2(1):e200033.
44. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425-434.
45. Wong KT, Antonio GE, Hui DSC, et al. Thin-section CT of severe acute respiratory syndrome: Evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228(2):395-400.
46. Ye Z, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. March 2020:1-9.
47. WHO. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Interim Guid. 2020;(March):1-7.
48. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020.
49. Suh CH, Kim KW, Pyo J, et al. The incidence of ALK inhibitor-related pneumonitis in advanced non-small-cell lung cancer patients: A systematic review and meta-analysis. Lung Cancer. 2019;132:79-86.
50. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2017;377(9):829-838.
51. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29-39.
52. Koshikawa K, Terada J, Abe M, et al. Clinical characteristics and risk factors of drug-induced lung injury by ALK tyrosine kinase inhibitors: A single center retrospective analysis. Thorac Cancer. April 2020.
53. Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK -rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(12):1683-1696.
54. Yoneda KY, Scranton JR, Cadogan MA, et al. Interstitial Lung Disease Associated With Crizotinib in Patients With Advanced Non–Small Cell Lung Cancer: Independent Review of Four PROFILE Trials. Clin Lung Cancer. 2017;18(5):472-479.
55. Suh CH, Park HS, Kim KW, et al. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients. Lung Cancer. 2018;123:60-69.
56. Jänne PA, Yang JC-H, Kim D-W, et al. AZD9291 in EGFR Inhibitor–Resistant Non–Small-Cell Lung Cancer. N Engl J Med. 2015;372(18):1689-1699.
57. Goss G, Tsai C-M, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17(12):1643-1652.
58. Terbuch A, Tiu C, Moreno Candilejo I, et al. Radiological patterns of drug induced interstitial lung disease (DILD) in early phase oncology clinical trials. Clin Cancer Res. 2020:clincanres.0454.2020.
59. Suresh K, Naidoo J, Lin CT, et al. Immune Checkpoint Immunotherapy for Non-Small Cell Lung Cancer. Chest. 2018;154(6):1416-1423.
60. ASCO Coronavirus Resources | ASCO. https://www.asco.org/asco-coronavirus-information. Accessed November 10, 2020.
61. COVID-19 and Cancer | ESMO. https://www.esmo.org/covid-19-and-cancer. Accessed November 10, 2020.
62. About NCCN. https://www.nccn.org/covid-19/default.aspx. Accessed November 10, 2020.
63. Banna G, Curioni-Fontecedro A, Friedlaender A, et al. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5(2):e000765.
64. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395(10229):1033-1034.
65.Figuero-Pérez L, Olivares-Hernández A, Escala-Cornejo RA, et al. Management of Febrile Neutropenia Associated With SARS-CoV-2 Infection in a Patient With Cancer. JCO Oncol Pract. 2020;16(6):348-349.
66.Taha M, Sharma A, Soubani A. Clinical deterioration during neutropenia recovery after G-CSF therapy in patient with COVID-19. Respir Med Case Reports. 2020;31:101231.
67. Lasagna A, Zuccaro V, Ferraris E, Rizzo G, Tancredi RJ, Pedrazzoli P. How to Use Prophylactic G-CSF in the Time of COVID-19. JCO Oncol Pract. September 2020:OP.20.00484.
68. Alkan A, Uncu A, Taşkıran I, Tanrıverdi Ö. Double-edged sword: Granulocyte colony stimulating factors in cancer patients during the COVID-19 era. Clinics. 2020;75:1-2.
69. Wang Z, Wang J, He J. Active and Effective Measures for the Care of Patients with Cancer during the COVID-19 Spread in China. JAMA Oncol. 2020.
70. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clic Med. 2020;0(0):100331.
71. Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on cancer outcomes of the COVID-19 pandemic. medRxiv. April 2020:2020.04.21.20073833.
72. Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21(1):515-546.
73. Li G, Chen X, Xu A. Profile of Specific Antibodies to the SARS-Associated Coronavirus. N Engl J Med. 2003;349(5):508-509.
74. Woo PCY, Lau SKP, Wong BHL, et al. Longitudinal Profile of Immunoglobulin G (IgG), IgM, and IgA Antibodies against the Severe Acute Respiratory Syndrome (SARS) Coronavirus Nucleocapsid Protein in Patients with Pneumonia Due to the SARS Coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665.
75. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020:2020.03.30.20047365.
76. Qin C, Zhou L, Hu Z, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;53(9):1689-1699.
77. Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic Testing for Severe Acute Respiratory Syndrome–Related Coronavirus-2. Ann Intern Med. 2020;10.7326/M20-1301
Received: June 03, 2020;
Accepted: June 11, 2020;
Published:June 25, 2020.
To cite this article : Briviesca RL, Calvo V, Bermúdez AC, et al. Lung Cancer and COVID-19: Therapeutic Implications. European Journal of Respiratory Medicine. 2020: 2:1.
©Provencio M, et al. 2020..
