Research Article/ Open Access
DOI:10.31488/EJRM.127
Management of Covid-19 based on Risk Features
Fernando Cabanillas*1,5, Javier Morales2, José G. Conde3, Jorge Bertrán-Pasarell1,5, Ricardo Fernández4,5, Yaimara Hernandez-Silva5, Idalia Liboy5, James Bryan-Díaz1,5, Juan Arraut-Gonzalez5, Margarita Bruno-Padilla5
1.University of Puerto Rico School of Medicine, Puerto Rico
2.Clinical Research Puerto Rico, Puerto Rico
3.University of Puerto Rico Medical Sciences Campus, Puerto Rico
4.San Juan Bautista School of Medicine, Puerto Rico
5.Auxilio Mutuo Hospital, Puerto Rico
*Corresponding author: Fernando Cabanillas, MD, Auxilio Mutuo Hospital, Puerto Rico
Abstract
Covid-19 is a triphasic disorder first typified by a viral phase that lasts from first onset of symptoms until seven days later. This is followed in 20% by respiratory failure usually heralded by an elevation of inflammatory markers. The aims of this study were: 1- To determine feasibility of identifying low-risk patients who can be safely monitored at home without treatment. 2- To identify patients at high risk of progressing to hypoxemic respiratory failure so they can be treated preemptively with methylprednisolone. Eligible were those 21 years or older with oxygen saturation >91%. For patients to be classified as high-risk, they had to exhibit two or more of the following abnormalities 7-10 days after first symptom: IL-6 > 10 pg/ml, ferritin > 500 ng/ml, D-dimer > 1 mg/L, CRP > 10 mg/dL, LDH above normal, lymphopenia, oxygen saturation 91-94%, or positive CT chest. CALL score was used to predict expected number of cases of respiratory failure. High risk patients received methylprednisolone (MPS) 80 mg IV daily x 5 starting no earlier than seven days from onset of symptoms. Primary endpoint was respiratory failure. Change in levels of inflammatory markers and length of hospitalization were also assessed. None of 132 low risk cases developed respiratory failure. In 76 high risk patients, the expected number with respiratory failure was 30 (39.5%), yet only 4 (5.3%) developed that complication (p=.00001). Improvement in inflammatory markers strongly correlated with a favorable outcome. Our results are encouraging and suggest this approach is both effective and safe.
Keywords: Methylprednisolone, Covid-19, CRP, LDH, IL-6, absolute lymphocyte count, inflammatory markers, cytokine storm, respiratory failure
Introduction
Covid-19 is a triphasic disorder first typified by a viral phase that lasts from the first onset of symptoms until approximately 7 days later. This is followed by a second phase considered as the inflammatory stage, characterized first by the appearance of lung infiltrates, which can result in hypoxemia [1-4]. This second phase is usually heralded by an elevation of serologic inflammatory markers such as C-reactive protein (CRP), ferritin, interleukin-6 (IL-6) [5], LDH (lactic dehydrogenase) as well as D-dimers [4,6,7]. In a smaller subset of cases this is followed by a third phase consisting of hyperinflammation that leads to the cytokine release syndrome or cytokine storm, which causes Acute Respiratory Distress Syndrome (ARDS) [1,2]. Mortality secondary to Covid-19 is usually related to this latter complication.
Approximately 20% of patients will proceed to the second phase. This rate is a crude estimate that can vary according to several prognostic factors [3,4,7]. Currently, there is no reliable and objective method to accurately predict the 80% that are cured spontaneously without any treatment, vis-à-vis those 20% who develop severe illness. There is consensus that those presenting with severe illness characterized by hypoxemia, should be managed in the inpatient setting and treated with dexamethasone [8]. However, according to the RECOVERY trial, only patients with severe illness requiring oxygen administration in the hospital, benefit from that treatment. Those who are non-oxygen dependent, not only fail to benefit, but could actually be harmed by the use of dexamethasone [8].
We hereby report on the results of a prospective clinical trial designed with two goals in mind:
1. To determine if it is feasible to prospectively identify early during their illness, a group of low-risk patients who could be safely monitored at home without any treatment.
2. To prospectively identify non-oxygen-dependent patients with moderately severe Covid-19 but at high-risk of progressing to hypoxemic respiratory failure. Our aim was to evaluate in this second group of patients, the effect of preemptive treatment with corticosteroids to prevent them from developing cytokine release syndrome.
We prospectively divided Covid-19 patients by ranking them into high and low-risk, according to blood-based biomarkers of inflammation, as well as other clinical features shown below.
Materials and Methods
Investigational plan
The study was registered in clinicaltrials.gov as NCT04355247 and approved by the local Institutional Review Board (IRB) at Auxilio Mutuo Hospital in San Juan, Puerto Rico on April 13, 2020. The informed consent form approved by the local IRB was discussed with each patient and signed prior to registration. The study was conducted at Auxilio Mutuo Hospital in San Juan, Puerto Rico. Patients were recruited from San Juan and any other city in Puerto Rico between April and December 2020, consequently none of the patients on study were vaccinated at time of entry to study. The first patient was entered on April 19, 2020.
The original plan was to enter a total of 100 patients with the expectation that at least 20 would-be high-risk cases eligible for therapy with MPS and 80 would be low-risk, eligible for monitoring without therapy. This expectation was based on the available literature data, which describes a 20% chance for patients with Covid-19 to develop severe disease associated with respiratory failure [3,6]. We later decided to expand this pilot study to include at least 200 patients.
Eligibility
Eligible patients were those 21 years or older with a diagnosis of Covid-19 established by means of either the PCR molecular test (97% of cases registered) or with the rapid serologic test in the context of typical symptoms and/or ground glass infiltrates in the chest CT (3% of cases). There was no top age limit for entry.
Excluded from entry were those already in acute respiratory failure defined as oxygen saturation <90%. Other exclusion criteria included anyone who was chronically oxygen dependent, or who had long standing history of severe COPD (chronic obstructive pulmonary disease). Patients receiving tocilizumab, convalescent plasma, or prednisone 20 mg daily or more, or equivalent, prior to entering the study, were also excluded.
Definition of high-risk cases
For patients to be classified as high-risk, they had to exhibit two or more of the following abnormalities between days 7-10 after their first symptom: IL-6 > 10 pg/ml, ferritin > 500 ng/ml, D-dimer > 1 mg/L (1,000 ng/ml), CRP > 10 mg/dL (100 mg/L), LDH above normal range, lymphopenia (absolute lymphocyte count), oxygen saturation between 91-94%, or CT chest with evidence of ground glass infiltrates. These markers were selected based on published data which have shown a strong association with outcome [3,4,7]. When the CALL score method to predict prognosis was published [9], we amended the protocol to apply this score with the purpose of allowing a more precise estimate of the expected number of cases that would develop respiratory failure. The CALL Score method considers the presence of comorbidities, age, LDH level and lymphopenia to assign a prognostic score [9]. The higher the score, the worse the prognosis. It includes a nomogram which can be used to predict each patient’s risk of progression to respiratory failure. The investigator assessing the outcome was unblinded to assignment of treatment.
Definition of low-risk cases
Anyone not fitting the definition of high risk was classified as low risk. We also analyzed the symptomatology at presentation to determine if low-risk patients could be identified by the number of symptoms at diagnosis or by the type of presenting symptoms.
Therapy
Management of low-risk cases
Patients classified as low-risk were monitored at home without treatment. They were asked to check their oxygen saturation three times per day by means of pulse oximetry and to report any value less than 94% during the first two weeks after entry. They were also instructed to immediately report any unexpected change in their clinical condition. Patients were also called daily to inquire about their condition. Outpatients were also contacted daily by the data manager for 28 days to inquire about their condition. The data manager entered the data in an Excel database spreadsheet.
Management of high-risk cases
Treatment consisted of methylprednisolone (MPS) 80 mg IV daily x 5 days starting no earlier than 7 days from first onset of symptoms. Intravenous route was selected to ensure that doses were given as ordered and that absorption was not an issue. Treatment was never started within the first 6 days of illness to avoid prolonging the viral phase because of delay in clearance of the virus induced by MPS. Later, the protocol was amended to allow use of a higher dose in morbidly obese patients who were given 160 mg daily x 5 days. This was done in two patients.
Patients were similarly asked to check their oxygen saturation three times per day by means of pulse oximetry and to report to the data manager any value less than 94%.
Simultaneous treatment with the following drugs was not encouraged but was allowed: hydroxychloroquine, azithromycin, vitamin C, doxycycline, colchicine, zinc and ivermectin. Treatment with tocilizumab was not allowed at time of initiation of MPS but in those cases responding sub-optimally after the third day of MPS, the protocol did not preclude its use (more details below). After the 6th patient was registered, we started delivering MPS treatments at the patient’s household by means of a home-health care agency, provided they were stable enough.
Of the 21 patients who required hospitalization, 16 of these were already hospitalized at the time therapy with MPS was started, because they either had mild hypoxemia or required management of some other Covid complication or co-morbidity already existent at the time they were entered in the study. There were another five admitted following outpatient therapy because of respiratory failure in two, dyspnea without respiratory failure in another two, and profound weakness in one. Four of the 21 cases were admitted to the medical intensive care unit (MICU).
Outcome measures
Primary endpoint was progression to hypoxemic respiratory failure defined as an oxygen saturation of < 90% or p02 <60.
Secondary endpoints included:
1. Survival at 28 days from registration.
2. Admission to medical intensive care unit (MICU).
3. Live discharge from the hospital.
4. In addition, measurement of inflammatory markers in high-risk cases was repeated at day 7 of the study in order to compare with pre-treatment values, and length of hospital admission was also calculated.
Changes in each of the inflammatory markers of high-risk cases were classified into two major groups:
• Favorable changes:
• normal Pre MPS to normal Post MPS
• high pre MPS to normal Post MPS
• for lymphopenia, the ALC (absolute lymphocyte count) favorable changes were classified as
1.normal Pre MPS to normal Post MPS
2.low Pre MPS to normal Post MPS.
Unfavorable changes
• High Pre MPS to high Post MPS
• normal pre MPS to high Post MPS
• for lymphopenia, the ALC unfavorable changes were classified as
• low Pre MPS to low Post MPS
• normal Pre MPS to low Post MPS.
Statistical analysis
Medians and interquartile ranges (IQR’s) were used to describe distributions of continuous variables, and proportions to describe distributions of categorical variables. IQR’s are reported as lower and upper limits of the IQR (i.e., first and third quartiles of distributions). This format not only provides information on the IQR, but also indicate location of the central 50% of distributions relative to the median. The Wilcoxon-Mann-Whitney test was used for testing hypotheses about the difference between distributions of continuous variables, and Fisher’s exact chi-square test for categorical variables. The pairwise sign test was used to assess changes in proinflammatory markers after treatment. The binomial test was used to compare the number of observed cases of respiratory failure to the number expected as predicted by the CALL Score [10].
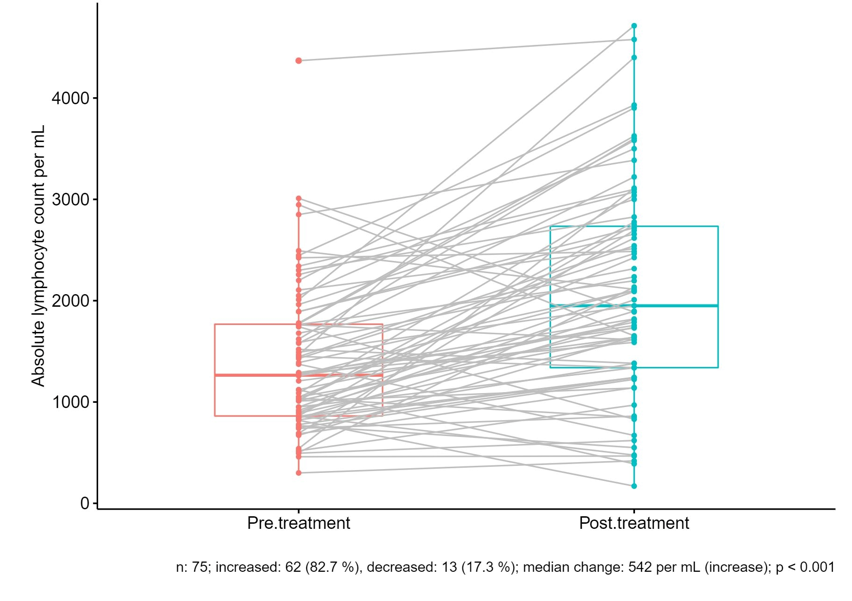
Figure 1:Change in ALC After Treatment with Methylprednisolone
Results
We initially enrolled 213 patients (Figure 1), 5 of which were not evaluable because consent was withdrawn (n=3), PCR for Covid-19 was negative (n=1), and no blood markers were performed due to suicidal attempt (n=1). Of the 208 evaluable cases, 76 were categorized as high-risk. Median follow-up for the high-risk cases was 3,116 person days (range 1,140-10,488), IQR was 2,622 with 25th percentile =34 and 75th percentile=74.25. No patients were lost to follow up. Results were analyzed using the intention to treat principle. Anyone who received at least one dose of MPS was considered evaluable.
Table 1 depicts the demographics of the whole sample including low as well as high-risk cases. Male gender was more commonly represented in high-risk patients, but this did not reach statistical significance (p=0.36). High-risk patients were significantly older than low-risk. Similarly, the number of comorbidities was significantly greater in the high-risk cases. The CALL score correlated well with the risk score with most low-risk cases corresponding to the less advanced CALL score “A group” and only 2.2% were high-risk patients, however 47% fell into the intermediate risk score category B. Likewise, high-risk cases presented more commonly with lower oxygen saturation. Regarding symptoms, fever and dyspnea were more commonly seen in high-risk cases.
Table 1:Demographics at Baseline
| Feature | Low risk (N=132) | % | High-risk (N=76) | % | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 62 | 47 | 40 | 52.6 | 0.36 |
| Female | 70 | 53 | 36 | 47.3 | |
| Median age (IQR*) | 45 (35.5,5) | - | 60 (50,72) | - | 0.00001 |
| Median # comorbidities per patient (IQR) | 1 (0,1) | - | 1 (1,2) | - | 0.00001 |
| CALL Score | |||||
| 4-6 (Class A) | 64 | 48.5 | 14 | 18.4 | 0.0001 |
| 7-9 (Class B | 62 | 47 | 36 | 47.3 | |
| 10-13 (Class C) | 3 | 2.2 | 26 | 34.2 | |
| Missing information | 3 | 2.2 | 0 | 0 | |
| O2 saturation 91-94% | 2 | 1.5 | 23 | 30.2 | 0.00001 |
| Symptoms: | |||||
| Fever | 49 | 37.1 | 48 | 63.2 | 0.0001 |
| Dyspnea | 21 | 15.9 | 29 | 38.2 | 0.004 |
| Myalgia | 84 | 63.6 | 49 | 64.4 | 1.0 |
| Diarrhea | 55 | 41.7 | 33 | 43.4 | 0.88 |
| Anosmia | 67 | 50.7 | 34 | 44.7 | 0.47 |
The presence of dyspnea and pneumonia are frequently used as criteria for inpatient management. There were 48 of our low-risk patients who had a CT chest done, of which 17 had typical findings of ground-glass infiltrates and would have been hospitalized. Of these, 5 had dyspnea and 12 did not. An additional 9 cases had a negative CT chest but had dyspnea. Another 10 cases had dyspnea without hypoxemia, hence a CT chest was not done.
Clinical outcome low risk cases
None of the 134 low-risk cases developed respiratory failure and none developed any type of complication that required admission to the hospital (95% CI 0 to 0). None of them died and none received any rescue medications except for two who developed pulmonary emboli and received anticoagulation.
Clinical outcome according to CALL Score
Respiratory failure
After applying the CALL Score to the 76 high-risk patients, the expected number of cases of respiratory failure was 30 (39.5%). However, after treatment with MPS, only 4 (5.3%) developed that complication (p=.00001). When CALL score was applied to all cases including low-risk and high-risk features, 206 cases had all the information necessary to calculate the score and the expected number of cases of respiratory failure rate was 43 (20.9%) while the observed rate was 4 (1.9%), (p<.00001).
Tocilizumab was administered to two patients who after 5 days of MPS had not improved satisfactorily regarding their symptomatology or oxygen saturation without respiratory failure. This is not considered as a protocol deviation because treatment with Tocilizumab was not allowed prior to entering the study but it was not forbidden in cases not responding well to methylprednisolone. It was also given to another two of four patients who had already been counted as respiratory failures. This decision was done in combination with the IL-6 pre-treatment levels which were elevated at 182.8, 70.0, 65.2 and 12.26 pg/ml. All four improved clinically within 24 hours, although one later deteriorated and died. In an additional three cases, treatment with MPS was extended beyond five days due to suboptimal improvement in fever or dyspnea without respiratory failure. This is considered as a deviation from protocol therapy.
Difference between markers pre and post MPS
The change in all markers studied pre and post MPS is shown in table 2. There was a statistically significant difference in CRP, LDH, IL-6, ferritin and absolute lymphocyte count (ALC) before and after treatment. Figure 1 portrays the pre and post MPS changes in ALC in each case. In 17 cases the increase in ALC resulted in complete resolution of lymphopenia.
Table 2:Change in Markers (Post-Treatment with Methylprednisolone minus Pre-Treatment)
| Marker | Median difference (IQR) |
Total patient | Increased N (%) |
Decreased N (% |
p-value |
|---|---|---|---|---|---|
| CRP (mg/dL) | -0.85 (-5.20, 0.08) |
73 | 19 (26.0) | 49 (67.1) | < 0.001 |
| LDH units | 44 (-100.75, 2.25) |
72 | 19 (26.4) | 53 (73.6) | < 0.001 |
| IL-6 (pg/ml) | -9.00 (-15.74, 0.10) |
66 | 15 (22.7 | 49 (74.2) | < 0.001 |
| Ferritin (ng/ml) | -57.3 (-174.3, 0.4) |
71 | 18 (25.4) | 52 (73.2) | < 0.001 |
| D-dimer (mg/L) | 0.02 -0.51, 0.41) |
69 | 30 (43.5) | 36 (52.2) | 0.54 |
| ALC per mL |
542 (169, 1,102) |
75 | 62 (82.7) | 13 (17.3) | < 0.001 |
Survival, admission to MICU and live discharges
None of the patients in the low risk group required admission to the hospital. There was one death within 28 days of registration on study (in high risk group), for a 28-day survival rate of 98.6%. Another patient in the high risk group died after day 28 (on day 57) because of complications related to COVID-19. There were five patients who required admission to MICU at some point during their illness, all of them in the high risk group.
Once we started delivering treatment at the patient’s household, which took place eight days after the protocol was initiated, a total of 14 required hospitalization, nine at the time they were first seen and five others who started treatment at home but had to be admitted subsequently. Twelve were discharged alive after a median of 10 days of hospitalization while the other two died in the hospital.
Changes in blood markers and their association with clinical outcome
We examined the association between clinical outcome and change in pre- to post-treatment values of pro-inflammatory blood markers as well as with ALC. Table 3 portrays the various patterns from pre to post MPS levels and their association with clinical outcome, defined as number of unfavorable events or endpoints which included death, admissions to MICU and respiratory failure.
There was a statistically significant association between CRP, LDH, D-dimers and IL-6 (Table 3) and the combined unfavorable endpoints. However, the most noticeable association observed was with the post treatment changes in ALC. When the ALC pattern was from normal to normal or low to normal, only 4.7% of 63 cases had an unfavorable outcome while there were 66.6% of 12 cases who met at least one of the unfavorable endpoints if the pattern was from low to low or normal to low (p<.0001).
A pre-treatment ALC <1,000 has been previously associated with a high rate of respiratory failure [9], so we attempted to confirm this finding. Table 4 examines the association between various levels of pre-treatment ALC with respiratory failure and shows that in contrast with the difference between pre and post treatment ALC, there is no statistically significant association at any of the levels examined.
Toxicity
The only serious adverse reaction observed was in two diabetic patients (one type II and the other one type I), whose blood sugar while on MPS increased to the point that an addition or increase of insulin was necessary. Otherwise, MPS was well tolerated.
Discussion
High risk cases
The use of corticosteroids in the management of Covid-19 has been criticized because of the legitimate concern regarding the use of immunosuppressive medications that may cause a delayed viral clearance and increase the risk of secondary infections. However, the data available against the use of steroids [11] was derived in great extent from trials in which they were used either too early during the viral phase, thus placing patients at risk of worsening their infection, or too late, at which time their efficacy was compromised.
After recognizing the role of excessive inflammation as an important factor in the pathogenesis of respiratory failure in Covid-19, dexamethasone was successfully introduced and tested in the RECOVERY trial for hospitalized patients [8]. Later, high dose MPS [12] was used in the Netherlands by Ramiro et al in a clinical trial designed exclusively for the management of an established cytokine storm. As shown in these two trials, corticosteroids are effective. However, in cases with advanced presentations, the mortality can be reduced but is still considerable [8,12].
In early 2020, Dr. Angel Atienza, from Hospital Doctor Peset, Valencia, Spain proposed the use of a brief course of MPS early during the illness, right at the end of the viral phase, and shortly before the second or inflammatory phase of the disease (personal communication). The aim was to utilize it as preventive therapy for patients at high risk of entering the second phase. He proposed to apply it to non-oxygen dependent cases with high-risk clinical features identified by certain defined abnormalities in their blood parameters. In the RECOVERY trial, these oxygen-independent patients failed to benefit and possibly were harmed by the 10 day course of dexamethasone [8]. Yet our findings in oxygen-independent cases treated preemptively with MPS are encouraging. These data strongly suggest that this novel preemptive approach is not only effective but also safe. It is not clear how many of these oxygen-independent patients in the RECOVERY trial received dexamethasone during the first seven days of illness which could help explain their poor outcome.
According to the published medical literature [4,13], the number of cases with Covid-19 expected to develop respiratory failure is roughly 20%, which is equivalent to 42 of the 208 patients entered on our study. This closely matches the projection of 43 expected cases estimated by applying the CALL score. In the 76 high-risk cases we treated, a respiratory failure rate of 39.5% was expected according to their CALL score, but the observed rate was much lower, 5.3% (p<.00001). None of the 132 low-risk cases developed respiratory failure. We conclude that this approach has the potential of averting the cytokine storm commonly seen in this disorder, when applied to high-risk cases after day 7-10, but never earlier than 7 days after the first symptom.
Particularly interesting was the association we observed between clinical outcome and changes in pre-treatment CRP, LDH, D-dimers and IL-6 compared with the day 7 post MPS values (Table 3), which suggests that MPS might have reduced inflammation, and as result decreased the number of Covid related complications, including respiratory failure.
Table 3:Association between change I n Blood Parameters at Day 7 Post MPS treatment and clinical endpoints
| Change in CRP from pre-treatment to post-treatment | Total died, respiratory failure or MICU admission | |||
|---|---|---|---|---|
| Yes | No | Total | p | |
| Normal->normal or high->normal | 0 (0) | 45 (100%) | 45 | 0.00001 |
| High->High or Normal->high | 10 (35.7%) | 18 (64.3%) | 28 | |
| Change in LDH from pre-treatment to post-treatment | Yes | No | Total | P |
| Normal->normal or high->normal | 2 (3.9%) | 49 (96%) | 51 | 0.0005 |
| High->High or Nor- 0.0005 mal>High | 8 (38.1%) | 13 (61.9% | 21 | |
| Change in Ferritin from pre-treatment to post-treatment | Yes | No | Total | P |
| Normal->normal or | 1 (3.6%) | 27 (196.4%) | 28 | 0.14 |
| high->normal | ||||
| High->High or | 7(16.3%) | 36 (83.7%) | 43 | |
| Normal->high | Change in D-dimers from pre-treatment to post-treatment | Yes | No | Total | P |
| Normal->normal or | 0 (0) | 28 (100%) | 28 | 0.004 |
| high->normal | ||||
| High->High or | 10(24.4%) | 31 (75.6%) | 41 | |
| Normal->high | Change in IL-6 from pre-treatment to post-treatment | Yes | No | Total | P |
| Normal->normal or | 2 (3.8%) | 51 (96.2%) | 53 | 0.009 |
| high->normal | ||||
| High->High or | 4 (33.3%) | 8 (66.7%) | 12 | |
| Normal->high | Change in ALC* from pre-treatment to post-treatment | Yes | No | Total | P |
| Normal->normal or | 3 (4.7%) | 60 (95.2%) | 63 | < 0.00001 |
| low->normal | ||||
| Low->Low or | 8 (67%) | 4 (33.3%) | 12 | |
| Normal->Low | ||||
*ALC= Absolute Lymphocyte Count
The weak association we observed between pre-treatment ALC and development of respiratory failure (Table 4), although unexpected, perhaps should not be surprising. It is a well-known fact that when newer and more effective treatments are introduced, these can alter or totally abolish well-established prognostic factors [14].
Table 4:Association between Various Pre-treatment ALC Levels and Clinical Outcome
| ALC /µL at Baseline | N=76 | Respiratory Failure | P value |
|---|---|---|---|
| ≥ 500 | 73 | 3 (3.9%) | 0.15 |
| < 500 | 3 | 1 (33.3%) | |
| ≥ 750 | 64 | 3 (4.6%) | 0.15 |
| < 750 | 12 | 1 (8.3%) | |
| ≥ 1000 | 50 | 2 (4%) | 0.6 |
| < 1,000 | 26 | 2 (7.7%) | 0.6 |
ALC= absolute lymphocyte count
Pre-treatment refers to day 7-10 post first symptom.
day 7 CBC was not done in 1 patient.
The increase in ALC observed in 63 cases is consonant with our interpretation that treatment with MPS could have brought about this improvement which correlated well with a lower rate of respiratory failure, while those whose day 7 ALC post-MPS failed to improve, or dropped below normal, tended to fare less well. It is unclear why MPS, which is a lympholytic agent, is capable of increasing the peripheral blood absolute lymphocyte count. One possibility is that these lymphocytes are being trapped in the lungs in areas of inflammation. After the anti-inflammatory effects of MPS, they might be released into the bloodstream.
A similar pattern of improvement in markers was observed on day 7 post MPS levels of CRP, LDH, D-dimers, and IL-6 (Table 3), again suggesting a beneficial effect of this anti-inflammatory medication.
Low-risk cases
Included in the low-risk group as defined by our criteria, were 69 cases with comorbidities, Covid-19 pneumonia (n=17) as well as with other adverse features including age >65 (n=15), and multiple symptoms at presentation (n=98). These features would usually portend a poor prognosis according to traditional standards, yet all of our low-risk cases had an excellent outcome.
The management of mild to moderate Covid-19 infections has traditionally been conservative. Patients with either Covid-19 pneumonia or with dyspnea, are usually admitted to the hospital (15). Otherwise, treatment has consisted of observation at home provided there is no evidence of hypoxemia. Applying these traditional criteria to decide on hospitalization, 26 of our 48 low-risk patients who had a CT chest, would have been managed as inpatients (5 of them because of dyspnea with positive chest CT, 9 with dyspnea but negative CT chest and 12 with no dyspnea but positive CT chest). An additional 10 cases had dyspnea, but a CT chest was not done. This adds up to a total of 36 patients who traditionally would have been managed as in-patients. By applying our novel criteria to identify low-risk cases, we were able to safely manage all these 36 patients at home without disassociating them from their family. Furthermore, assuming an average of 10 days of hospitalization per patient, it allowed us to save an average of 360 days of hospitalization. At an average cost of hospitalization of $3,949 per day, this adds up to a total of $14,216,400 US dollars saved. In addition, these 36 patients were prevented from exposure to nosocomial infections.
Conclusions
Our management approach, based on blood-based inflammatory markers as well as other clinical features, had an excellent correlation with the clinical outcome. Possible confounding factors in our results include the use of tocilizumab in two patients and the additional doses of MPS in another three cases who were not responding as expected. However, even if we do not consider these five cases as successfully treated, the results still would favor MPS since a total of 9 (11.8%) would then be considered as failures compared with the 30 expected cases (39.5%), p=.0002.
Although the data generated by this exploratory trial appear strong and compelling, confirmation of these findings in an independent study would be highly desirable.
Funding Sources:
1. Puerto Rico Coalition for Clinical Investigation which provided support for data management.
2. This project was partially funded by RCMI Grant U54 MD007600, NIMHD, NIH. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
Best Option Company provided treatment with methylprednisolone at patients’ households free of cost. The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests and no conflicts of interests.
Author Contributions
FC contributed to the conception of the study, analyzed and interpreted the patient data regarding the treatment and outcome. JM was a major contributor in conception of the study, in managing the patients and writing the manuscript. YHS contributed to acquisition of data and its management. JGC contributed to the statistical analysis of data. All others contributed to the design and analysis of data. All authors read and approved the final manuscript.
References
1. Magro G. COVID-19: Review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020;286:198070.
2. Khadke S, Ahmed N, Ahmed N, et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol J. 2020;17(1):154.
3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-62.
4. Mughal MS, Kaur IP, Jaffery AR, et al. COVID-19 patients in a tertiary US hospital: Assessment of clinical course and predictors of the disease severity. Respir Med. 2020;172:106130.
5. Liu T, Zhang J, Yang Y, et al. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019; medRxiv. 2020:2020.03.01.20029769.
6. Zhou J, He W, Liang J, et al. Association of interleukin-6 level with morbidity and mortality in patients with coronavirus disease 2019 (COVID-19). Jpn J Infect Dis. 2020.
7. Bastug A, Bodur H, Erdogan S, et al. Clinical and laboratory features of COVID-19: Predictors of severe prognosis. Int Immunopharmacol. 2020;88:106950.
8. Group RC, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020; Epub ahead of print. .
9. Ji D, Zhang D, Xu J, et al. Prediction for Progression Risk in Patients with COVID-19 Pneumonia: the CALL Score. Clin Infect Dis. 2020.
10. Zar J. Biostatistical Analysis. 2nd ed. Hall P, editor. New Jersey1984 1.
11. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-5.
12. Ramiro S, Mostard RLM, Magro-Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79(9):1143-51.
13. Hussain A, Kaler J, Tabrez E, et al. Novel COVID-19: A Comprehensive Review of Transmission, Manifestation, and Pathogenesis. Cureus. 2020;12(5):e8184.
14. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857-61.
15. Macera M, De Angelis G, Sagnelli C, et al. Clinical Presentation of COVID-19: Case Series and Review of the Literature. Int J Environ Res Public Health. 2020;17(14).
Received: January 27, 2022;
Accepted: February 15, 2022;
Published: February 17, 2022.
To cite this article : Cabanillas F, Morales J, Conde JG, et al. Management of Covid-19 based on Risk Features. European Journal of Respiratory Medicine. 2022; 4(1): 276-284. doi: 10.31488/EJRM.127.
© 2022 Cabanillas F, et al..
