Research article/ Open Access
DOI:10.31488/EJRM.134
Mental Health Disorders After Mild COVID-19: INCREASE IN Fatigue and Depression – A Substudy of the Single-Center Controlled Follow-Up Study of COVID-19 in The District of Constance (FSC19-KN)
Sabrina Geng1, Jonas Haberland1, Elisabeth Haberland1, Stephan Richter1 MD, Micheal Schmid1 MD, Julia Hromek1 MD, Heidi Zimmermann1, Hannes Winterer2 MD, Steffen Schneider3 PhD, Michael Jöbges4 MD PhD, Marc Kollum 1 MD PhD
1. Hegau Bodensee Klinikum Singen, Gesundheitsverbund Landkreis Konstanz, Virchow Str. 10, 78224 Singen, Germany
2. Landratsamt Konstanz, Amt für Gesundheit und Versorgung – Gesundheitsamt, Scheffelstraße 15, 78315 Radolfzell, Germany
3. Institut für Herzinfarktforschung, Bremserstr. 79, 67063 Ludwigshafen, Germany
4. Kliniken Schmieder Konstanz, Neurologisches Fach- und Rehabilitationskrankenhaus, Eichhornstraße 68, 78464 Konstanz, Germany
*Corresponding author: Dr. Marc Kollum PhD, Hegau-Bodensee Klinikum Singen, I. Medizinische Klinik Virchow Str. 10, 78224 Singen, Germany, Tel: +49 7731 89 2600; Fax: +49 7731 89 2605
Abstract
Importance: An increasing prevalence of mental health disorders such as fatigue, depression and anxiety has been observed since the beginning of the COVID-19 pandemic. However, they might not only be caused by infection itself but also by pandemic circumstances. Objective: To investigate the incidence of symptoms of fatigue, depression, and panic disorder in SARS-CoV-2 positive subjects in comparison to a control cohort. Design: A controlled follow-up of mild COVID-19 cases in the district of Constance, Germany. Setting: This monocentric population-based trial is designed as a sub-study of the Follow-up Study of COVID-19 in the district of Constance (FSC19-KN). Participants: 280 SARS-CoV-2 PCR positive subjects were recruited as a random sample in cooperation with the local health department. 238 subjects with negative SARS-CoV-2 antibody titers were recruited as a volunteer sample in the main study and requested to and requested to participate in the substudy subsquently. Exposure: Infections in the SARS-CoV-2 positive group occurred between March and December 2020. Main Outcome and Measures: The presence of fatigue, depression and panic disorder was evaluated in both groups via two questionnaires: Fatigue Scale for Motor and Cognitive Functions (FSMC) and Patient Health Questionnaire (PHQ-9/-PD). Results: Among 376 subjects 216 were female (57.4%), mean age was 49.4 years. 192 (96.5%) of the SARS-CoV-2 positive participants had a mild disease course without hospitalization. The General Fatigue Score was significantly higher in the SARS-CoV-2 positive group (mean ± standard deviation 42.8±20.2 vs. 32.1±15.7 controls; difference in means 10.7; 95% CI 7.0 to 14.4). General fatigue symptoms were detected more frequently in the SARS- CoV-2 positive group (OR 3.76; 95% CI 2.10 to 6.90). The PHQ-9 score was significantly higher in the SARS-CoV-2 positive group (5.6±4.9 vs. 3.2±3.9 controls; difference in means 2.4; 95% CI 1.5 to 3.3). Clinically relevant depressive symptoms were significantly more common in the SARS-CoV-2 positive group (OR 3.0; 95% CI 1.45 to 6.55). Conclusion: This is aggravated by the fact that the etiology although most subjects had a mild disease course without hospitalization, clinically relevant symptoms of fatigue and depression were recorded more than twice more frequently in the SARS-CoV-2 positive group.
Background
The chronic fatigue syndrome has a long history. There are many theories concerning the etiology of the chronic fatigue syndrome like induction by viral infections, endocrine disorders, autoimmune diseases, cancer, and multiple sclerosis [1-6] .
The prevalence of fatigue varies from 0.3% up to 25% [7,8]. The variation in prevalence is due to frequently changing diagnostic criteria, case definitions, heterogenous study collectives and complex constellations of symptoms [9,10]. The etiology and pathophysiology of the chronic fatigue syndrome has not been fully resolved yet.
Fatigue-related symptoms like post-exertional malaise, cognitive dysfunction, unrefreshing sleep, pain and autonomic dysfunction (e.g. dizziness and fainting upon standing up, inability to alter heart rate with exercise, sweating abnormalities) indicate that this disorder is related to central nervous system [11].
Structural and functional neuroimaging has shown significant reductions in global grey matter volume in chronic fatigue syndrome, as well als a decreased connectivity in fatigue patients several brain areas [12-14].
Furthermore, the identification of disease specific objective biological parameters is a current goal of research. The chronic fatigue syndrome has been associated with associated with higher levels of C-reactive Protein, reduced cortisol levels and elevated cytokines [15-17].
In the Fatigue Scale for Motor and Cognitive Functions (FSMC) which is commonly applied to multiple sclerosis patients there is a differentiation between motor and cognitive fatigue [18]. But many studies concerning patients with fatigue symptoms exclude patients who suffer from depression [19,20]. Contradictorily, it has been shown that major depressive disorders or mood disorders often correlate with chronic fatigue syndromes [21,22]. Nevertheless, frequently applied diagnostic classifications for Chronic Fatigue Syndrome such as the 1994 International Research Case Definition, 2003 Canadian Consensus, 2011 ME International Consensus regard psychiatric disorders as exclusion criteria for the diagnosis of a fatigue syndrome [23-25]. In Germany a common term is "chronic fatigue syndrome" while ''myalgic encephalomyelitis'' and chronic fatigue syndrome are commonly used in international literature in international literature. Since no objective method to diagnose a fatigue syndrome has been established so far, it is a diagnosis by exclusion despite its presumably high prevalence.
Respiratory virus infections (e.g. by human respiratory syncytial virus, by human metapneumovirus, by influenza) have been observed to cause neurologic alterations. In particular, neuroinvasive properties have been found in coronaviruses, such as SARS-CoV-2 [26,27]. A systematic international review and meta-analysis showed an incidence of acute encephalitis as a complication of COVID-19 was 0.2% [28]. In the COVID-19 pandemic a long-term impact on patients has been observed. In this connection fatigue was one of the most frequently reported symptoms [29,30]. In a Chinese cohort study a 6-month observation after SARS-CoV-2 infection had shown that patients suffer from ongoing mental disorders like anxiety and depression [31]. This connection aroused the suspicion that fatigue could be a long-term effect of SARS-CoV-2-infection.
A study on the gerneral US populatiion has proven a 3-fold higher pravalence of depressive symptoms during the COVID-19-pandemic than before [32]. This is in accordance with observations among residents in Taiwan after the SARS crisis in 2003 about 9.2% of the participants who reported that their perceptions of life became more pessimistic during the pandemic even without having been directly affected by the disease [33].
Unfortunately several studies regarding mental health disorders and fatigue were conducted either in post-COVID patients or in the general population during the pandemic [31,32]. Therefore, the question could not been answered so far whether the increased incidences of mental disorders or fatigue is due to a previous SARS-CoV-2 infection or pandemic circumstances like e.g. isolation measures, contact restrictions and lockdowns.
The sub-study at hand aims to address this gap in understanding by examining both a group of post-COVID subjects and a control cohort.
Methods
Study design
The study at hand was designed as a sub-study of the FSC19-KN [34]. It was conducted as a monocentric cohort study in a controlled setting. Its main objective was to investigate the incidence of mental health disorders in SARS-CoV-2 positive subjects living in the local district of Constance (Baden Wurttemberg, Germany). Approval was given by the ethics committee of the Albert-Ludwigs-University (Freiburg). The study was registered on the German Clinical Trials Register and Clinicaltrials.gov.
Participants
In the main study FSC19-KN 281 participants had a Polymerase-Chain-Reaction (PCR)-confirmed SARS-CoV-2 infection and 238 controls showed negative SARS-CoV-2 antibody titers [34]. All subjects were contacted and asked to participate in our sub-study (Figure 1).
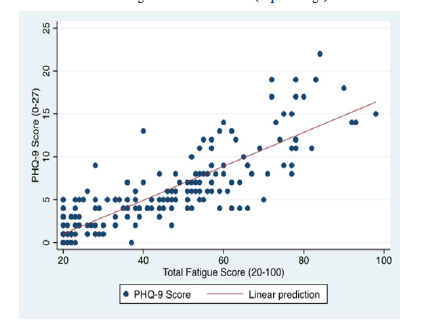
Figure 1:Graph 1. Correlation between the FSMC-score and PHQ-9-score
The presence of fatigue (Fatigue Scale for Motor and Cognitive Functions, FSMC), depression and anxiety disorder (Patient Health Questionnaire, PHQ-9/-PD) was evaluated via online questionnaires or by post using paper versions.
Questionnaires
In multiple sclerosis patients fatigue is well known and has a high prevalence up to 78.0% as a secondary illness [35]. It can be further divided into cognitive or motor fatigue [36]. The FSMC is highly sensitive and specific in detecting fatigue in multiple sclerosis patients and its internal consistency and reliability are high [37].
The FSMC general fatigue score (20-100) categorizes none to mild (score < 53) and moderate to severe (score ≥ 53) fatigue. Furthermore, the cognitive fatigue score (10-50) categorizes none to mild (score < 28) and moderate to severe cognitive fatigue (score ≥ 28), whereas the motor fatigue score (10-50) categorizes none to mild (score < 27) and moderate to severe (score ≥ 27) motor fatigue.
The Patient Health Questionnaire-9 (PHQ-9) comprises nine questions concerning depression. Its total score (0-27) is used to determine the degree of depression (none to severe) [38].
The PHQ–9 is a validated survey for major depression with a sensitivity of 88% and a specificity of 88% at a cutoff score of 10 or higher [38]. The score categorizes none to mild (score < 10) and moderate to severe (score ≥ 10) depression. The latter category is considered clinically relevant. Additionally, in clinical practice a score greater than ten corresponds to the possible diagnosis of a major depression and gives reason to either initiate therapy or to follow a watch and wait-procedure [38,39].
The Patient Health Questionnaire – Panic Disorder (PHQ-PD) is a subsection of the PHQ-9 and comprises 15 questions on panic disorder symptoms [40].
Since both questionnaires overlap in terms of content (e.g., concentration, tiredness), we checked a possible correlation between the general fatigue score of the FSMC- and PHQ-9-score results.
Ethical consideration
The task of filling out the questionnaires can be stressful for subjects with preexisting mental disorders and lead to an aggravation of their psychological state. To address this problem personal consultation, assistance completing the questionnaires and further information on possible contact addresses were provided to the participants if necessary.
Statistical analysis
Descriptive statistics were used for a comparative presentation of sociodemographic data. All statistical analyses were performed with STATA. FSMC and PHQ-9/-PD score results are given as mean ± standard deviation (SD). Differences in means (DM) and their respective 95% confidence intervals (CI) were calculated via t-test for independent samples with pooled variances [41]. The strength of the association between two events was quantified by odds ratios (OR). The respective 95%-confidence intervals were determined by log odds ratio function [42]. Missing values were not included during data analysis but recorded accurately. To determine a correlation between fatigue and depression a correlation co-efficient was calculated according to Bravais-Pearson.
All study data were collected and managed using a Research Electronic Data Capture (REDCap) platform hosted at https://redcap.glkn.de [43,44]. The accuracy of the data entries was verified by an external monitor according to guidelines for good clinical practice
.Results
Study population
The mean time from PCR testing to survey in September 2021 was 341±89 days. Only 7 subjects (3,5%) of the SARS-CoV-2 positive subjects were hospitalized and 1 subject (0,5%) was ventilated mechanically and monitored in an intensive care unit.
Age, gender distribution and cardiovascular risk factors did not differ in the two groups (Table 1). The mean age of the SARS-CoV-2 positive participants was 49.0±15.3 years (range 18 to 80) and the mean age of the controls was 49.7±14.1(range 18 to 78) years. A total of 112 of the SARS-CoV-2 positive subjects (56.3%) and 104 of the controls (58.8%) were female.
Table 1: Distribution of cardiovascular risk factors in study population
| SARSCoV-
2
positive n=199 |
Control n=177 |
Missing
Value SARS-CoV2 positive/ SARS-CoV2 negative |
|
|---|---|---|---|
| Age – years | 49.0±15.3 | 49.7±14.1 | 0 / 0 |
| 18-39 years – no. (%) | 56 (28.1) | 40 (22.6) | 0 / 0 |
| 40-59 years – no. (%) | 99 (49.7) | 92 (52.0) | 0 / 0 |
| 60-79 years – no. (%) | 43 (21.6) | 45 (25.4) | 0 / 0 |
| ≥ 80 years – no. (%) | 1 (0.5) | 0 (0.0) | 0 / 0 |
| Gender | |||
| Male – no. (%) | 87 (43.7) | 73 (41.2) | 0 / 0 |
| Female – no. (%) | 112 (56.3) | 104 (58.8) | 0 / 0 |
| Cardiovascular Risk Factors | |||
| Diabetes Mellitus – no. (%) | 4 (2.0) | 5 (2.8) | 0 / 0 |
| Arterial Hypertension – no. (%) | 38 (19.1) | 26 (14.7) | 0 / 0 |
| Hypercholesteremia – no. (%) | 26 (13.3) | 17 (9.6) | 4 / 0 |
| Smoking – no. (%) | 79 (39.7) | 64 (36.2) | 0 / 0 |
| Family History of CAD – no. (%) | 31 (15.9) | 26 (14.9) | 4 / 2 |
Data are given as absolute value (in percentage)/ Mean ± Std. Dev.
Regarding the distribution of cardiovascular risk factors like diabetes mellitus, hypercholesteremia, smoking and family history of coronary artery disease, there was no difference between both study groups
Primary endpoint
The summarized results of the FSMC were given in table 2. The mean general fatigue score was significantly higher in the SARS-CoV-2 positive group (42.8±20.2 vs. 32.1±15.7 controls; DM 10.7; 95% CI 7.0 to 14.4).
Table 2: Number of Fatigue, Scores by using the FSMC
| SARS-CoV2
positive n=199 |
Control Proband n=177 |
Odds Ratio | Difference in Means | Missing Value SARS-CoV-2 positive / controls |
|
|---|---|---|---|---|---|
| Total Fatigue Score (20-100) | 42.8 ± 20.22 | 32.06 ± 15.17 | - | 10.7±1.9 [7.0 to 14.4] |
6 / 2 |
| Moderate – Severe total Fatigue Score ≥ 53 – no. (%) |
63 (32.6) | 20 (11.4) | 3.76 [2.10 to 6.90] |
- | |
| Cognitive Fatigue Score (10-50) | 21,39 ± 10,42 | 15.97 ± 7.77 | - | 5.4 [3.5 to 7.3] |
5 / 2 |
| Moderate – Severe cognitive Fatigue Score ≥ 28 – no. (%) |
52 (26.8) | 19 (10.9) | 3,01 [1.65 to 5.64] |
- | |
| Motor Fatigue Score (10-50) | 21,54 ± 10,26 | 16,09 ± 7,71 | - | 5.5 [3.6 to 7.3] |
3 / 2 |
| Moderate – Severe motor Fatigue Score ≥ 27 – no. (%) |
65 (33.2) | 21 (12.0) | 3.63 [2.06 to 6.60] |
- |
Data are given as absolute value (in percentage)/ Mean ± Std. Dev. and in square brackets 95% CI
Table 3: Number of Depression and Panic Disorder, Scores by using PHQ-9 and PHQ-PD
| SARS-CoV2
positive n=199 |
Control
Proband n=177 |
Odds Ratio | Difference in Means | Missing Value SARS-CoV-2 positive / Controls |
|
|---|---|---|---|---|---|
| PHQ-9 Score (0-27) | 5.61 ± 4.85 | 3.18 ± 3.85 | - | 2.4 [1.5 to 3.3] |
0 / 2 |
| Moderate - Severe Depression Score ≥ 10 – no. (%) |
36 (18.1) | 12 (6.9) | 3.0 [1.45 to 6.55] |
- | |
| PHQ-PD (Panic Disorder) | 0 / 2 | ||||
| No Panic Syndrome – no. (%) | 191 (96.0) | 174 (99.4) | 2.4 [0.6 to 9.3] |
Data are given as absolute value (in percentage)/ Mean ± Std. Dev. and in square brackets 95% CI
Fatigue symptoms were more common in the SARS-CoV-2 positive group as compared to the controls (OR 3.76; 95% CI 2.10 to 6.90). The mean cognitive fatigue score was significantly higher in the SARS-CoV-2 positive group (21.4±10.4 vs. 16.0±7.8 controls; DM 5.4; 95% CI 3.5 to 7.3). Cognitive fatigue symptoms were more common in the SARS-CoV-2 positive group (OR 3.01; 95% CI 1.65 to 5.64). The mean motor fatigue score was significantly higher in the SARS-CoV-2 positive group (21.5±10.3 vs.16.1±7.7 controls; DM 5.5; 95% CI 3.6 to 7.3). Relevant motor fatigue symptoms were significantly more common in the SARS-CoV-2 positive group (OR 3.63; 95% CI 2.06 to 6.60).
Secondary endpoints
The mean PHQ-9 score was significantly higher in the SARS-CoV-2 positive group (5.6±4.9 vs. 3.2±3.9 controls; DM 2.4; 95% CI 1.5 to 3.3). Clinically relevant symptoms were significantly more common in the SARS-CoV-2 positive group (OR 3.0; 95% CI 1.45 to 6.55); Table 3. Symptoms of a panic syndrome could not be detected significantly more often in the SARS-CoV-2 positive group.
Correlation
The strength and direction of the relationship between the general fatigue score and the phq-9-score s is demonstrated in the graph 1. It shows a strong positive correlation with a correlation coefficient R=0.72 between the two scores.
Discussion
This sub-study proves that even eight to fourteen months after a mild disease course of COVID-19 a considerable number of SARS-CoV-2-positive subjects suffer from symptoms corresponding to mental health disorders (depression and fatigue) in comparison to control subjects.
Comparing the overall prevalence of depressive symptoms in Germany (PHQ-8 score > 10) before the pandemic in the GEDA 2014/2015-EHIS study to the prevalence of depressive symptoms (PHQ -9 score > 10) in the control group, the latter one appeared to be even lower despite pandemic circumstances since march 2020 [45]. This observation contradicts study results from Denmark showing that the local population felt affected negatively in their psychological well-being by the COVID-19 pandemic [46].
Depressive symptoms were detected more than twice more often, and general symptoms of fatigue were detected almost three times more often in the SARS-CoV-2 positive group.
The results could suggest that a higher prevalence of symptoms of fatigue and depression is related to a previous SARS-CoV-2-infection, but COVID-19 may not be the only factor responsible for this observation.
Possibly the awareness of the infection itself and its possible severe consequences could influence the SARS-CoV-2 positive group to such an extent that they report psychological symptoms significantly more often.
Both cognitive and motor fatigue scores are higher in the SARS-CoV-2 positive group. The association between SARS-CoV-2 infection and motor fatigue appears larger than for cognitive fatigue. This indicates that the fatigue after COVID 19 infection is rather motoric than cognitive. The correlation analysis to illustrates a clear association of fatigue and depression in the SARS-CoV-2 positive group. Neuroimaging proceduces such as structural and functional neuroimaging could be used to further explore this correlation and its underlying pathomechanism.
Cardiovascular, pulmonary and neurological sequelae of COVID-19 have been identified before [47-49]. Since post-COVID patients especially suffer from symptoms like fatigue, headache and attention disorder, the differential diagnosis of a mental health disorder should be considered more often in these patients [50].
Since most studies published so far observed long-term consequences over short periods of time, more long-term observation of COVID-19-patients is needed [47-50].
Limitation
A selection bias may be present, since subjects suffering from high degree depression or fatigue are more likely to deny study participation [51] and therefore may have excluded themselves from the substudy a priori, thus even leading to an underestimation of the findings at hand. On the other hand, the investigated subjects provided a description of their thoughts, feelings, or behaviors referring their mental health via questionnaires, which could lead to self-report bias [52].
Due to the low hospitalization rate in the SARS-CoV-2 positive cohort the findings are only applicable to mild courses. The study results, however, affect the majority of infected patients, since more than 90% of the COVID patients in Germany were not hospitalized in 2021 [53].
Furthermore, the PHQ-9 is recommended in a two-stage screening process [54]. Therefore a follow-up examination is planned in 2022.
Conclusion
Although most SARS-CoV-2 positive subjects had a disease course without hospitalization, clinically relevant symptoms of fatigue and depression were observed significantly more often in the SARS-CoV-2 positive group. Since the study data were acquired in a controlled setting, the results may suggest that the higher prevalence of mental health disorders could be associated with the previous infection.
To identify the exact mechanisms leading to increasing prevalence of mental disorders and persistence of mental health disorders during the global COVID-19 pandemic further research is required. The adjustment of diagnostic and therapeutic procedures in post-COVID patients should be conducted based on further significant data.
Key Points
Question: Is there an increased prevalence of fatigue and depression after COVID-19 in comparison to a control cohort?
Findings: In this controlled follow-up study which included 209 SARS-CoV-2 PCR positive subjects and 183 subjects with negative SARS-CoV-2 antibody titers, the symptoms of fatigue were detected almost three times and depressive symptoms twice more frequently in the SARS-CoV-2 positive group.
Meaning: A higher prevalence of symptoms of fatigue and depression is associated with SARS-CoV-2 infection and is not only due to pandemic circumstances
Trial Registration
clinicaltrials.gov Identifier: NCT04883190
German Clinical Trials Register Identifier: DRKS00025176
References
1. Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. doi:10.1136/bmj.38933.585764.AE
2. Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med. 2001;31(8):1331-1345. doi:10.1017/S0033291701004664
3. Komaroff AL, Buchwald, MD DS. Chronic Fatigue Syndrome: An Update. Annu Rev Med. 1998;49(1):1-13. doi:10.1146/annurev.med.49.1.1
4. Smets E, Garssen B, Schuster-Uitterhoeve A, et al. Fatigue in cancer patients. Br J Cancer. 1993;68(2):220-224. doi:10.1038/bjc.1993.319
5. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of Cancer-Related Fatigue. The Oncologist. 2007;12(S1):22-34. doi:10.1634/theoncologist.12-S1-22
6. Freal JE, Kraft GH, Coryell JK. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1984;65(3):135-138.
7. Bates DW, Schmitt W, Buchwald D, et al. Prevalence of Fatigue and Chronic Fatigue Syndrome in a Primary Care Practice. Arch Intern Med. 1993;153(24):2759-2765. doi:10.1001/archinte.1993.00410240067007
8. Cullen W, Kearney Y, Bury G. Prevalence of fatigue in general practice. Ir J Med Sci. 2002;171(1):10-12. doi:10.1007/BF03168931
9. Lim EJ, Ahn YC, Jang ES, et al. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18(1):100. doi:10.1186/s12967-020-02269-0
10. Afari N, Buchwald D. Chronic Fatigue Syndrome: A Review. Am J Psychiatry. 2003;160(2):221-236. doi:10.1176/appi.ajp.160.2.221
11. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4). doi:10.1515/reveh-2015-0026
12. de Lange FP, Kalkman JS, Bleijenberg G, et al. Gray matter volume reduction in the chronic fatigue syndrome. NeuroImage. 2005;26(3):777-781. doi:10.1016/j.neuroimage.2005.02.037
13. Wylie GR, Yao B, Genova HM, et al. Using functional connectivity changes associated with cognitive fatigue to delineate a fatigue network. Sci Rep. 2020;10(1):21927. doi:10.1038/s41598-020-78768-3
14. Chen MH, Wylie GR, Sandroff BM, et al. Neural mechanisms underlying state mental fatigue in multiple sclerosis: a pilot study. J Neurol. 2020;267(8):2372-2382. doi:10.1007/s00415-020-09853-w
15. Spence VA, Kennedy G, Belch JJF, et al. Low-grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome. Clin Sci. 2008;114(8):561-566. doi:10.1042/CS20070274
16. Papadopoulos AS, Cleare AJ. Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol. 2012;8(1):22-32. doi:10.1038/nrendo.2011.153
17. Fletcher MA, Zeng XR, Barnes Z, et al. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7(1):96. doi:10.1186/1479-5876-7-96
18. Penner I, Raselli C, Stöcklin M, et al. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler J. 2009;15(12):1509-1517. doi:10.1177/1352458509348519
19. Wessely S, Chalder T, Hirsch S, et al. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health. 1997;87(9):1449-1455. doi:10.2105/AJPH.87.9.1449
20. Vincent A, Brimmer DJ, Whipple MO, et al. Prevalence, Incidence, and Classification of Chronic Fatigue Syndrome in Olmsted County, Minnesota, as Estimated Using the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(12):1145-1152. doi:10.1016/j.mayocp.2012.08.015
21. Abbey SE, Garfinkel PE. Chronic Fatigue Syndrome and Depression: Cause, Effect, or Covariate. Clin Infect Dis. 1991;13(Supplement_1):S73-S83. doi:10.1093/clinids/13.Supplement_1.S73
22. Lane TJ, Manu P, Matthews DA. Depression and somatization in the chronic fatigue syndrome. Am J Med. 1991;91(4):335-344. doi:10.1016/0002-9343(91)90150-V
23. Fukuda K. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann Intern Med. 1994;121(12):953. doi:10.7326/0003-4819-121-12-199412150-00009
24. Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J Chronic Fatigue Syndr. 2003;11(1):7-115. doi:10.1300/J092v11n01_02
25. Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270(4):327-338. doi:10.1111/j.1365-2796.2011.02428.x
26. Bohmwald K, Gálvez NMS, Ríos M, et al. Neurologic Alterations Due to Respiratory Virus Infections. Front Cell Neurosci. 2018;12:386. doi:10.3389/fncel.2018.00386
27. Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by Human Respiratory Coronaviruses. J Virol. 2000;74(19):8913-8921. doi:10.1128/JVI.74.19.8913-8921.2000
28. Siow I, Lee KS, Zhang JJY, et al. Encephalitis as a neurological complication of COVID‐19: A systematic review and meta‐analysis of incidence, outcomes, and predictors. Eur J Neurol. 2021;28(10):3491-3502. doi:10.1111/ene.14913
29. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: A cross‐sectional evaluation. J Med Virol. 2021;93(2):1013-1022. doi:10.1002/jmv.26368
30. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi:10.1056/NEJMoa2005412
31. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397(10270):220-232. doi:10.1016/S0140-6736(20)32656-8
32. Ettman CK, Abdalla SM, Cohen GH, et al. Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Netw Open. 2020;3(9):e2019686. doi:10.1001/jamanetworkopen.2020.19686
33. Peng EYC, Lee MB, Tsai ST, et al. Population-based Post-crisis Psychological Distress: An Example From the SARS Outbreak in Taiwan. J Formos Med Assoc. 2010;109(7):524-532. doi:10.1016/S0929-6646(10)60087-3
34. Kollum PD med M. Single Center Prospective Controlled Follow-up Study of COVID-19 Patients in the District Konstanz (FSC19-KN).
35. Oliva Ramirez A, Keenan A, Kalau O, et al. Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. BMC Neurol. 2021;21(1):468. doi:10.1186/s12883-021-02396-1
36. Sehle A, Neumann M, Spiteri S, et al. Fatigue und kognitive Beeinträchtigungen bei der MS. neuroreha. 2014;06(01):22-28. doi:10.1055/s-0034-1372453
37. Penner I, Raselli C, Stöcklin M, et al. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler J. 2009;15(12):1509-1517. doi:10.1177/1352458509348519
38. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
39. Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29(5):388-395. doi:10.1016/j.genhosppsych.2007.06.004
40. Bernd Löwe, Robert L. Spitzer, Stephan Zipfel, et al. PHQ-D : Gesundheitsfragebogen für Patienten ; Manual Komplettversion und Kurzform. 2. Auflage. Karlsruhe Pfizer GmbH; 2002. https://www.klinikum.uni-heidelberg.de/fileadmin/Psychosomatische_Klinik/download/PHQ_Manual1.pdf
41. Weiß C. Basiswissen medizinische Statistik. 7., vollständige und überarbeitete Auflage. Springer; 2019. doi:10.1007/978-3-662-56588-9
42. Bland JM. Statistics Notes: The odds ratio. BMJ. 2000;320(7247):1468-1468. doi:10.1136/bmj.320.7247.1468
43. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010
44. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
45. Bretschneider J, Kuhnert R, Hapke U. Depressive Symptomatik bei Erwachsenen in Deutschland. 2017;2(3). doi:10.17886/RKI-GBE-2017-058
46. Sønderskov KM, Dinesen PT, Santini ZI, et al. The depressive state of Denmark during the COVID-19 pandemic. Acta Neuropsychiatr. 2020;32(4):226-228. doi:10.1017/neu.2020.15
47. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265. doi:10.1001/jamacardio.2020.3557
48. Zhao Y miao, Shang Y min, Song W bin, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi:10.1016/j.eclinm.2020.100463
49. Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020;77(8):1018. doi:10.1001/jamaneurol.2020.2065
50. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi:10.1038/s41598-021-95565-8
51. Patten SB. Selection bias in studies of major depression using clinical subjects. J Clin Epidemiol. 2000;53(4):351-357. doi:10.1016/S0895-4356(99)00215-2
52. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. Published online May 2016:211. doi:10.2147/JMDH.S104807
53. Schilling J, Tolksdorf K, Marquis A, et al. Die verschiedenen Phasen der COVID-19-Pandemie in Deutschland: Eine deskriptive Analyse von Januar 2020 bis Februar 2021. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2021;64(9):1093-1106. doi:10.1007/s00103-021-03394-x
54. Costantini L, Pasquarella C, Odone A, et al. Screening for depression in primary care with Patient Health Questionnaire-9 (PHQ-9): A systematic review. J Affect Disord. 2021;279:473-483. doi:10.1016/j.jad.2020.09.131
Received: June 03, 2022;
Accepted: June 20, 2022;
Published: June 23, 2022.
To cite this article : Geng S, Haberland J, Balensiefen E, et al. Mental Health Disorders After Mild COVID-19: INCREASE IN Fatigue and Depression – A Substudy of the Single-Center Controlled Follow-Up Study of COVID-19 in The District of Constance (FSC19-KN). European Journal of Respiratory Medicine. 2022; 4(2): 327 - 333. doi: 10.31488/EJRM.134.
© 2022 Geng S, et al.
