Research article/ Open Access
DOI:10.31488/EJRM.128
Mortality in Hospitalized Covid19 Patients during First Wave in a Tertiary Hospital in Northern Patagonia
Zabert Gustavo*1,2, Veltri Ignacio1,2, Zabert Ignacio1,2, Espinosa Lucio1, Mariluan Fabian1,2, Fernández Nelson1, Santini Marcela1, Alonso Marcelo1, Bullo Rosario1, Villa Josefina1, Goya Facundo1, Pincheira Carolina1, Calanni Liliana1, Figueroa Fernanda1, Carbajal Silvina1, Vaca Narvaja R1, Pelaez Juan1
1. Clínica Pasteur SA, Neuquén, Argentina
2. Facultad de Ciencias Medicas de la Universidad Nacional del Comahue, Argentina
*Corresponding author: Dr. Gustavo E Zabert, Clínica Pasteur, Rioja 26, 8300, Argentina
Abstract
In December 2019, an outbreak of respiratory disease caused by coronavirus, named SARS-CoV-2, was detected, and rapidly evolved to pandemic. The disease was characterized by high respiratory morbidity and mortality, and health systems were overwhelmed to assist an unusual number of patients with respiratory failure. In Argentina, quarantine and health control measures delayed the first pandemic peak and offered time to prepare the health system with infrastructure, personnel and protocols based on emerging evidence. To evaluate and adjust the institutional protocol of the Pasteur Clinic (Neuquén, Argentina), a non-interventional retrospective cohort study was designed with patient hospitalized for COVID-19 to determine global mortality during hospitalization and at 28th day of admission for the period 2020-2021. This report describes the results of the 501 patients diagnosed up to December 31, 2020. The observed mortality was 16.6% (83/501) and 12.2% (61/501) on day 28 of admission. Of the 139 (27.7%) patients who required mechanical ventilation, 37.4% (52/139) and 28.1% (38/139) died, respectively. The risk factors identified for both outcomes were age, comorbidities, and high oxygen requirements at admission. The mortality observed among hospitalized patients assisted under the Pasteur Clinic COVID-19´s protocol during the first wave was lower than national reports and, comparable to the international reports published for the same period.
Key words: COVID-19, outcomes, mortality rate
Introduction
In December 2019, an outbreak of severe pneumonia caused by a new coronavirus (SARS-CoV-2) was detected in Wuhan, China [1]. This highly contagious and deadly virus spread throughout Asia, Europe and progressively into the rest of the world population [2-6]. On March 4th, the first case was identified in Argentina and a week later, with 118,000 reported cases in 114 countries and more than 4,000 deaths, the WHO officially declared COVID-19´s pandemic [7].
The evolution in Asian and European countries foreseen a complex scenario for our region and country [8,9]. Countries with higher development and organization in health systems than our, registered a rapid saturation of critical care beds due to large number of patients with acute respiratory failure who required oxygen therapy and ventilatory support [4,5,10]. Consequently, the new and deadly pandemic imposed huge challenges of health providers services at all levels and required an innovative approach [11].
In March 2020, Pasteur Clinic´s Board decided to launch a crisis committee to develop and execute a contingency plan to adapt building, infrastructure, and personnel resources, to develop training programs and to adjust patient´s care and administrative processes to safely optimize assistance of COVID-19´s12 in a pandemic scenario that could exceed the institutional capacities as well as the regional health system entirely [9,13].
Finally, it was determined to perform interim evaluation of patients’ outcomes to adjust the contingency plan. In this report, we describe the mortality among patients affected by COVID-19´s hospitalized patients in Pasteur Clinic in the first wave of the pandemic.
Methods
An observational cohort study of all COVID19 patients admitted at Pasteur Clinic, a tertiary-level private hospital located in Neuquén city. The period of analysis for this report includes the last hospitalized patient diagnosed on December 31, 2020.
Participants included were severe or critical COVID19 cases defined by NIH, older than 18 years referred to admission by the attending or referring physician or CCC referral [14]. Acute SARS-CoV-2 infection was confirmed by direct methods from respiratory samples by real-time reverse transcriptase polymerase chain reaction (RT-PCRrt) and/or detection of viral antigen or clinical, radiology images and/or epidemiology compatible with COVID-19 or retrospectively by or SARS-CoV-2-specific IgM and/or IgG antibodies after day 14 of onset of symptoms.
Key exclusion criteria were mild or moderate cases (SpO2 >94%) and patients hospitalized for less than 24 hours and referred for diagnosis and/or severity evaluation or complications and convalescents from SARS-CoV-2 infection without evidence of a new infection event.
Primary outcome variables were death from any cause throughout hospitalization and mortality on day 28. Secondary endpoints explored in the interim analysis were factors associated with mortality, such as reported comorbidities, hypertension, diabetes, cancer, active smoking, chronic obstructive pulmonary disease (COPD) and obesity (BMI >30), oxygen requirement, mechanical ventilatory and extent of lesions on initial CT.
All patients were treated under the institutional protocol that considered diagnostic actions, isolation, timely allocation of resources for each case requirements, and clinical management based on patients’ needs and best available evidence at the time of hospitalization [15]. The protocol does not consider any intervention or procedure assigned for research purposes so that each patient received care according to the standardized criteria of the institution. Institutional protocol considered integration of all healthcare services in assistance teams conformed by medical doctors’ specialists, physiotherapists, nurses, psychologists and other health care providers and mandatory daily interaction at all levels to properly and timely allocate resources for admitted patients based on their needs [15]. Patients were admitted and transferred to higher level of support as early as possible assistance teams detected worsening in their course or complications. Institutional assistance and management protocols, with the main objective of early recognition and early intervention, were agreed and disseminated through mandatory training programs for personnel [12,16]. Furthermore, systematic epidemiological surveillance was implemented for health care personnel and patients.
Institutional procedures were periodically reviewed based on the evolution of the published evidence to be adjusted by consensus of the appropriate working group [17]. Also, the institution was incorporated to the COVID-19 Coordinator Center (CCC) network of Neuquén’s Ministry of Health. CCC ruled patient´s allocation daily based on basis bed availability and patient requirement, but each hospital-maintained independence to define institutional standard-of-care protocols. Clínica Pasteur´s ethics guidelines in the COVID19 pandemic scenario defined resource allocation based on “net benefit” criteria obeying equity, justice and non-discrimination principles and patients wills 18-21].
The study protocol was approved by the Teaching and Research Committee of the Pasteur Clinic, and by the Advisory Commission on Biomedical Research in Human Beings (CAIBSH) of Neuquén Province as an observational study (DI-2021-2641-E-NEU -SSLD#MS). The protocol was registered in clinicaltrials.gov (NCT05087394) and in the Argentinean National Health Research Registry (RIS N°31.00.21). No patient or investigator received any financial compensation or incentive for participating in this study.
The data was recorded synchronously in three databases (RedCap, DataTech and Excel) with different objectives, which were integrated, reviewed, and consolidated into a single registry for analysis. The protocol established periodic evaluations; therefore, this report corresponds to the interim analysis of all patients assisted along 2020. Quantitative variables were expressed according to their distribution, as means with standard deviation or medians with interquartile range, and categorical variables as percentage values. The continuous variables were compared according to the distribution, T-test was applied in those parametric and in the non-parametric Mann-Withney and χ2 in the categorical variables. STATA 15.1 software (StataCorp LLC, Texas, USA) was used for statistical analysis.
Results
Between March 23, 2020, and January 9th, 2021, 978 moderate, severe, and critical COVID-19 patients were assisted until discharge or death at Clínica Pasteur (Figure 1). 477 moderate cases (48%) were outpatients followed up by telemedicine, 11,4% (54) were admitted based on clinical deterioration and/or vital signs worsening or complications, no deaths were reported among ambulatory care. Among 501 admitted patients recruited 97.6% (489) were confirmed by RT-PCRrt, antigen test or serology, only twelve cases (2.4%) the diagnosis was accepted only on clinical-epidemiological criteria. No patient had received COVID19 vaccination jabs.
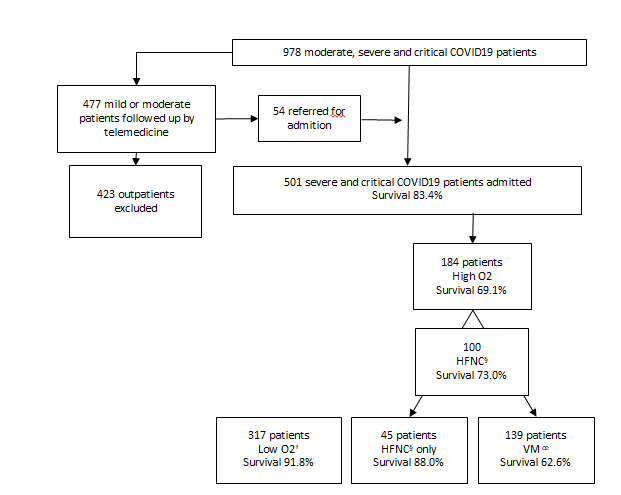
Figure 1:COVID-19 patients assisted at Clínica Pasteur, Neuquén, Argentina in 2020 (n = 978) † Low O2 oxigene by nasal cannula ‡ High O2: high-flow nasal cannula and/or mechanical ventilation § HFNC: high-flow nasal cannula oe MV: mechanical ventilation
Global hospital mortality and mortality on day 28 was 16.6% (83/501) and 12.2% (61/501), respectively. 94.4% (473/501) of the cases were admitted after June 1st and 49% (246/501) between October and November. The highest mortality was observed in November with 24.7% (20/81) of the total number of deaths (Figure 2) but in this interim analysis we found no difference in mortality by the date of admission (data not shown).
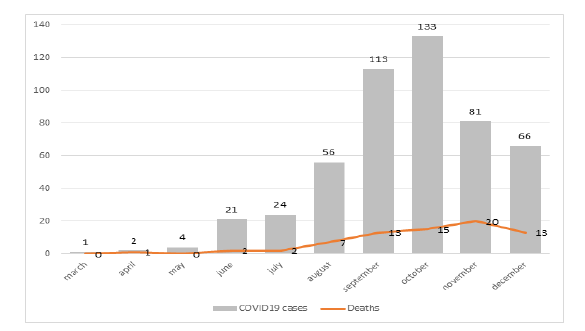
Figure 2:COVID-19 hospitalized patients and deaths registered between March 23rd, 2020, and January 9th, 2021, assisted at Clínica Pasteur, Neuquén, Argentina (n = 501)
Patient´s characteristics are shown in Table 1. 60.1% (301/501) were men and 78.6% (394/501) of patients reported comorbidities. The median age was 58 years (interquartile range 45-70 years) and the mean 57.4 years (SD 16.06 years). On admission, the most reported comorbidities were arterial hypertension (36.3%), obesity (29 .9%), and smoking (13.2%), while diabetes (8.5%), hypothyroidism (5.9%), cardiovascular disease (5.4%), dementia (5.4 %), asthma (4.2%), history of stroke (4.2%), cancer (3.4%) and COPD (3.6%). According to the Charlson score, 61.7% (309/501) cases were classified as without o low risk and 6% (30/501) as moderate and high risk., 18 (3.6%) admitted patients signed a limitation of therapeutic effort (LTE) to restrain mechanical ventilation if needed, and 1 of them revoked it during hospitalization.
Table 1:Demographic and clinical characteristics of COVID-19 hospitalized cases between March 23rd, 2020, and January 9th, 2021, assisted at Clínica Pasteur, Neuquén, Argentina (n = 501)
| n | % | |
|---|---|---|
| Male Gender | 301 | 60,1% |
| Mean Age (CI 95%) | 57,4 (56,1-58,8) | |
| Charlson´s Score | ||
| No risk | 309 | 61,7% |
| Low risk | 162 | 32,3% |
| Moderate risk | 14 | 2,8% |
| High risk | 16 | 3,2% |
| Comorbidities | 394 | 78,6% |
| Hypertension | 182 | 363% |
| Obesity | 149 | 29,9% |
| Active smoking | 66 | 13,2% |
| Diabetes | 108 | 8,5% |
| Hypothyroidism | 29 | 5,9% |
| Dementia | 27 | 5,4% |
| CV disease | 27 | 5,4% |
| Asthma | 22 | 4,3% |
| Stroke | 21 | 4,2% |
| Cancer | 17 | 3,4% |
| COPD | 15 | 3,0% |
| LTE* n (%) | 18 | 3,6% |
| Lung >50% (n total CT scan= 382) | 119 | 31,1% |
| High O2 n (%) | 184 | 36,7% |
| HFNC | 100 | 20,0% |
| MV | 139 | 27,7% |
*LTE: limitation of therapeutic effort
† Lung >50%: Chest CT at admission with lung involvement >50%
‡ High O2: high-flow nasal cannula and/or mechanical ventilation
§ HFNC: high-flow nasal cannula
oe MV: mechanical ventilation
Of the total number of patients, 317 (63.3%) received oxygen supply with low-flow systems, Venturi system or bag with reservoir and 184 (36.7%) required oxygen supplies by high-flow nasal cannula (HFNC) and/or mechanical ventilation (MV). All awake and hypoxemic patients were prone positioned by respiratory physiotherapists systematically as per protocol (data not shown).
Among 100 (20%) patients who initially receive HFNC, 55 (11%) progressed to MV and the remaining 45 (9%) patients overcame respiratory failure with HFNC without requiring further invasive procedures or orotracheal intubation. Non-invasive MV was indicated in only 2 patients, both with LTE that eventually died. 27.7% (139/501) of the cases required invasive MV, 55 (39.5%) of them initially received HFNC, and 2 patients progressed to extra-corporeal membrane oxygenation (ECMO). No patient had to be withheld due to scarce resources allocation.
Among patients (57/184) that required high oxygen requirements (HFNC and/or MV) mortality was significantly higher (30.9% vs 8.2% OR 5,02(IC95% 3.02-8.35), 52 of the 139 on MV (37.4% OR 6.02 CI 95% 3.41-10.63) and 27 of the 100 who received HFNC (27% OR 2.27 CI 95% 1.34-3.84). No mortality differences were observed between those who underwent HFNC prior to MV (39%) and those who were admitted directly to MV. Deaths among HFNC without MV was 11.1% (5/45) without significant differences with those who received low flow O2 (8.2% 26/317).
Pharmacological therapeutic interventions evolved on face with the published evidence. Of the 501 patients, all received anticoagulants at either prophylactic or therapeutic doses and only 20 patients were not prescribed corticosteroids, all of them hospitalized before June 22, 2020 (data not shown). Antibiotics were prescribed following institutional community pneumonia guidelines.
Following health regulation compassionate treatment was accepted according to the patients’ preferences in agreement with assistance physicians. 78 received convalescent plasma with no observed effect on hospital global mortality, at 28 days or less requiring MV (data not shown). The prescription of hydroxychloroquine and lopinavir/ritonavir was infrequent (<5%), all of them during the months of April and May. None of these cases had been vaccinated before admission.
Hospital mortality (Table 2) and at 28 days (Table 3) were associated with age (p<0.0001), presence of comorbidities (OR 8.82 CI95% 2.73-28.56 and OR 19.04 CI95 % 2.60-139.06), Charlson score (p<0.0001), high oxygen requirement (OR 5.02 CI95% 3.02-8.35 and OR 4.63 CI95% 2, 60-8.26) and lung involvement >50% extension at CT scan (OR 2.10 95% CI 1.10-4.01 and OR 2.26 95% CI 1.12-4.57). Setting apart high oxygen requirement in patients who required HFNC (OR 2.27 95% CI 1.35-3.84 and OR 2.00 95% CI 1.10-3.62) and MV (OR 6.38 CI95% 3.85-10.59 and OR 6.02 CI95% 3.41-10.64) both conditions were identified as risk factors for fatal outcome during hospitalization and on day 28. In this series, age was the variable with the highest risk for MV (61.3 years 95% CI 59.2-63.4 vs 56.1 years 95% CI 54.3-57.8 p= 0.001), for overall hospital death (70.5 years 95% CI 68.3-73.0 vs 54.9 years 95% CI 53.5-56.5 p<0.0001) and at 28 days (71.1 years CI 95% 68.1-74.2 vs 55.6 years 95% CI 54.2-57.1 p<0.0001).
Table 2:Hospital global mortality among COVID-19 hospitalized patients between March 23rd 2020 and January 9th 2021 assisted at Clínica Pasteur, Neuquén, Argentina (n = 501)
| Total | Deaths | Survivors | p | |
|---|---|---|---|---|
| Total, n (%) | 501 (100%) | 83 (16,6%) | 418 (83,4%) | |
| Gender | ||||
| Male n (%) | 301 (60,1%) | 57 (18,9%) | 244 (81,1%) | |
| Female n (%) | 200 (39,9%) | 26 (13,0%) | 174 (87,0%) | p NS |
| Age years X (SD) | 57,4 (16,0) | 70,6 (11,4) | 54,8 (15,5) | p< 0,0001 |
| Charlson score points X (SD) | 0,69 (1,33) | 1,40 (1,80) | 0,56 (1,20) | p< 0,0001 |
| Comorbidities n (%) | 394 (78,9%) | 80 (96,4%) | 314 (75,2%) | p< 0,0001 |
| Hypertension n (%) | 182 (36,3%) | 47 (53,6%) | 13 (32,3%) | p< 0,0001 |
| Obesity n (%) | 149 (29,9%) | 23 (27,7%) | 126 (30,1%) | p NS |
| Diabetes n (%) | 108 (13,2%) | 30 (36,1%) | 78 (18,7%) | p<0,0001 |
| Active smoking n (%) | 66 (8,5%) | 17 (20,5%) | 49 (11,7%) | p = 0,03 |
| Hypothyroidism n (%) | 29 (5,9%) | 9 (10,8%) | 20 (4,8%) | p =0,03 |
| Dementia n (%) | 27 (5,4%) | 9 (10,8%) | 18 (4,3%) | p =0,02 |
| CV disease n (%) | 27(5,4%) | 8 (9,6%) | 19 (4,6%) | p NS |
| Asthma n (%) | 22 (4,3%) | 2 (2,4%) | 20 (4,8%) | p NS |
| Stroke n (%) | 21 (4,2%) | 9 (10,9%) | 12 (2,9%) | p = 0,01 |
| Cancer n (%) | 17 (3,4%) | 7 (8,4%) | 10 (2,4%) | p = 0,005 |
| COPD | 15 (3,0%) | 7 (8,4%) | 8 (1,9%) | p = 0,004 |
| n (%) | ||||
| LTE* n (%) | 18 (3,6%) | 17 (20,5%) | 1 (0,2%) | p<0,0001 |
| Lung >50%† (n CT SCAN= 382) n (%) | 119 (31,1%) | 20 (46,5%) | 99 (29,2%) | p<0,021 |
| High O2 ‡ n (%) | 184 (36,7%) | 57 (68,7%) | 127 (30,4%) | p<0,0001 |
| HFNC§ n (%) | 100 (20,0%) | 27 (32,5%) | 73 (17,5%) | p<0,0002 |
| MV oe n (%) | 139 (27,7%) | 52 (62,6%) | 87 (20,8%) | p<0,0001 |
*LTE: limitation of therapeutic effort † Lung >50%: Chest CT at admission with lung involvement >50% ‡ High O2 : high-flow nasal cannula and/or mechanical ventilation § HFNC: high-flow nasal cannula oe MV: mechanical ventilation
Table 3:28th day hospital mortality among COVID-19 hospitalized patients between march 23rd, 2020 and January 9th 2021 assisted at Clínica Pasteur, Neuquén, Argentina (n = 501)
| Total | Deaths 28 days | Survivors | p | |
|---|---|---|---|---|
| Total, n (%) | 501 (100%) | 61 (12,8%) | 440 (87,8%) | |
| Gender | ||||
| Male n (%) | 301 (60,1%) | 39 (13,0%) | 262 (87,0%) | p NS |
| Female n (%) | 200 (39,9%) | 22 (11,0%) | 178 (89,0%) | |
| Age years X (SD) | 57,4 (16,0) | 70,9 (11,9) | 55,5 (15,6) | p< 0,0001 |
| Charlson score points X (SD) | 0,69 (1,33) | 1,31 (1,52) | 0,61 (1,29) | p< 0,0001 |
| Comorbidities n (%) | 394 (78,9%) | 60 (98,4%) | 334 (75,9%) | p< 0,0001 |
| Hypertension n (%) | 182 (36,3%) | 36 (59,0%) | 146 (33,2%) | p< 0,0001 |
| Obesity n (%) | 149 (29,9%) | 19 (31,28%) | 130 (29,6%) | p NS |
| Diabetes n (%) | 108 (13,2%) | 22 (36,1%) | 86 (19,5%) | p=0,003 |
| Active smoking n (%) | 66 (8,5%) | 12 (19,7%) | 54 (12,3%) | p NS |
| Hypothyroidism n (%) | 29 (5,9%) | 7 (11,5%) | 22 (5,0%) | p =0,04 |
| Dementia n (%) | 27 (5,4%) | 7 (11,5%) | 20 (4,5%) | p =0,03 |
| CV disease n (%) | 27(5,4%) | 7 (11,4%) | 20 (4,5%) | p =0,03 |
| Asthma n (%) | 22 (4,3%) | 2 (3,3%) | 20 (4,6%) | p NS |
| Stroke n (%) | 21 (4,2%) | 4 (6,6%) | 17 (3,9%) | p NS |
| Cancer n (%) | 17 (3,4%) | 5 (8,2%) | 12 (2,7%) | p = 0,03 |
| COPD n (%) | 15 (3,0%) | 7 (11,5%) | 8 (1,8%) | p <0,0001 |
| LTE* n (%) | 18 (3,6%) | 11 (18,0%) | 7 (1,5%) | p<0,0001 |
| Lung >50%† (n CT SCAN= 382) n (%) | 119 (31,1%) | 17 (48,6%) | 102 (29,4%) | P= 0,02 |
| High O2 ‡ n (%) | 184 (36,7%) | 42 (68,9%) | 142 (32,3%) | p<0,0001 |
| HFNC§ n (%) | 100 (20,0%) | 19 (31,2%) | 81 (18,4%) | P=0,02 |
| MV oe n (%) | 139 (27,7%) | 39 (63,9%) | 100 (22,7%) | p<0,0001 |
*LTE: limitation of therapeutic effort
† Lung >50%: Chest CT at admission with lung involvement>50%
‡ High O2: high-flow nasal cannula and/or mechanical ventilation
§ HFNC: high-flow nasal cannula
oe MV: mechanical ventilation
In univariate analysis, hypertension (OR 2.73 CI95% 1.69-4.42 and OR 2.89 CI95% 1.67-5.01), diabetes (OR 2.46 CI95% 1.48-4, 11 and OR 2.32 CI95% 1.30-4.11), cancer (OR 3.75 CI95% 1.38-10.17 and OR 3.18 CI95% 1.08-9.37), COPD (OR 4.72 CI95% 1.66-13.40 and OR 7.00 CI95% 2.44-20.06), dementia (OR 2.70 CI95% 1.16-6.24 and OR 2.72 CI95% 1.09-6.73) and hypothyroidism (OR 2.42 CI95% 1.06-5.52 and OR 2.46 CI95% 1.00-6.03) were associated with hospital and 28-day mortality. A significant relationship was observed between a history of stroke (OR 4.11 95% CI 1.67-10.11) and smoking with hospital mortality and cardiovascular disease (OR 2.72 95% CI 1.09-6.73) with death on day 28. Obesity was present in 29.9% of the population, but was not associated with mortality, analyzed as a dichotomous variable and when it was explored by BMI.
In the multivariate analysis using logistic regression models (Tables 4 and 5), age, history of comorbidities, and high oxygen requirements on admission were identified as factors associated with hospital and 28-day mortality.
Discussion
In this report we present the results in terms of mortality for severe and critical patients hospitalized in the first wave of the COVID-19´s pandemic in a private tertiary care center in Neuquén city adapted for readiness to address local pandemic scenario. With this aim, facilities, technological and human resources adjustments were performed before the pandemic impacted heavily in Argentina [8]. The peak incidence was concurrent with the evolution of the first wave in the region along 33 and 51 epidemiological weeks.
In our cohort, global hospital mortality and deaths measured at 28 days after admission were associated with older age, presence of comorbidities and extensive lung injure. In Neuquén Province, advanced age, comorbidities and MV requirement were associated with fatal outcome [9].
Reported comorbidities in our population were similar to those published by Schonfeld, et al. as well as in 2018´s Factors of Risk National Survey (ENFR), but differed in prevalence and mortality rate [22-26].
Schonfeld, et al. reported 207,079 cases of complete records in the Integrated System of Argentine Health Information (SIISA) from 738,776 cases diagnosed by RT-PCRrt database between May 3 and October 2, 2020 [22]. Mortality in hospitalized patients was between 23.0% (10,9135 deaths out of 43,355 hospitalized cases with complete data) and 28.0% (20,252 deaths out of 72,159 all hospitalized cases). Mean age was 55 years for those hospitalized in the general ward, 66 years for those in ICU and 74 years for those who died. Comorbidity prevalence was 75%, 86.1%, and 90.5% for the same groups, and hypertension (32.4%), diabetes (18.4%), and obesity (10.1%) were the most prevalent. In our series, comorbidities occurred in 78.6% of the cases, but diseases´ prevalence differed significantly from Schonfeld report.
Boietti, et al. published a report based on 4776 hospitalized patients between March 1 and October 31st, 2020, in 37 centers in Argentina, 70.2% located in metropolitan area and province of Buenos Aires´ hospitals [3]. Mean age was 56.9 years, 37% were older than 70 years, reported comorbidities were hypertension (32.4%), diabetes (15.4%), obesity (20.6%), and smoking (9.9%). In this series, 2,865 (62%) hospitalized patients did not required oxygen, 1,268 (27.4%) received low-flow nasal cannula oxygen, and 510 (10.6%) were required MV. The overall mortality reported was 12.3% and 54% among ICU admitted cases. In our cohort, the entire population required respiratory support, either low-flow oxygen therapy (63.3%) or HFNC and/or MV (36.7%), with an overall mortality of 16.6%, 8 .8% with low flow O2 systems and 37.4% in MV.
Estenssoro, et al. published 1,909 COVID-19 mechanical ventilated patients in 63 ICUs in Argentina between March 20 and October 31, 2020 [4]. Hospital global mortality and 28 days mortality for this series was 57.7% and 50.6% respectively. The population was characterized by a mean age of 62 years with a predominance of the male gender (67.8%) and a high prevalence of hypertension (46.9%), obesity (44.4%) and diabetes (29.0%). Only 7.5% of the cases received HFNC or NIV prior to MV without difference between surviving and deceased patients (7.2% vs 7.8% PNS).
Table 4:Logistic regression for hospital global mortality among COVID-19 hospitalized patients between March 23rd 2020 and January 9th 2021 assisted at Clínica Pasteur, Neuquén, Argentina (n = 501)
| Odds Ratio | Std. Err. | z | P>z | Intervalo Confianza 95% | |
|---|---|---|---|---|---|
| Age | 1,115,177 | 0,0212302 | 5,73 | 0,000 | 1,074333- 1,157573 |
| Male Gender | 2,231,992 | 0,9440152 | 1,90 | 0,058 | 0,9742693- 5,113357 |
| Comorbidities | 4,465,914 | 3,564,849 | 1,87 | 0,061 | 0,9342251- 21,34859 |
| Lung >50%† | 4,787,248 | 1,997,527 | 3,75 | 0,000 | 2,113069- 10,84572 |
| High O2 ‡ | 1,369,954 | 0,5422386 | 0,80 | 0,426 | 0,6306576- 2,975899 |
† Lung >50%: Chest CT at admission with lung involvement >50%
‡ High O2: high-flow nasal cannula and/or mechanical ventilation
Table 5:Logistic regression for 28th day hospital mortality among COVID-19 hospitalized patients between March 23rd, 2020, and January 9th, 2021, assisted at Clínica Pasteur, Neuquén, Argentina (n = 501)
| Odds Ratio | Std. Err. | z | P>z | Intervalo Confianza 95% | |
|---|---|---|---|---|---|
| Age | 1,127,706 | ,0247797 | 5,47 | 0,000 | 1,08017- 1,17335 |
| Male Gender | 179,466 | ,8122656 | 1,29 | 0,196 | 0,7391407- 4,357498 |
| Comorbidities | 7,210,737 | 7,767,837 | 1,83 | 0,067 | 0,8730004- 59,558965 |
| Lung >50%† | 5,070,843 | 2,347,308 | 3,51 | 0,000 | 2,04671-12,5633 |
| High O2 ‡ | 1,583,071 | ,6834612 | 1,06 | 0,287 | 0,6792194-3,689697 |
† Lung >50%: Chest CT at admission with lung involvement >50%
‡ High O2: high-flow nasal cannula and/or mechanical ventilation
Table 6:Comorbidities prevalence published by ENFR 2018 and in the publications of COVID-19 patients hospitalized in Argentina
| ENFR* | Schonfled y col 2 | Boietti | Estenssoro | Zabert | |
|---|---|---|---|---|---|
| 2018 1 | y col3 | y col 4 | y col | ||
| n | 49.170 | 47.4 | 4.8 | 1.9 | 501 |
| Hypertension | 34,7% | 35,6% | 32,4% | 46,9% | 36,3% |
| Diabetes | 12,7% | 18,4% | 15,8% | 29,0% | 8,5% |
| Obesity | 36,3% | 10,1% | 20,6% | 44,4% | 29,9% |
| Active smoking | 22,2% | 2,9% | 9,9% | 14,0% | 13,2% |
*ENFR Encuesta Nacional de Factores de Riesgo 2018, INDEC
Molini, et al. published 299 patients assisted with HFNC in 3 public hospitals of Neuquén´s Province [5]. Mean age was 65 years and 84.1% of patients without LTE reported comorbidities. The rate of progression to MV was 59.8% and the overall hospital mortality was 48.5%, 70.4% on MV and 4.16% for patients on HFNC only and excluded 47 who died in HFNC with LTE order. In our cohort, the overall mortality in patients who had high O2 requirements (HFNC and/or MV) was 30.9%, 37.4% mechanical ventilated patients, and 27% among all who used HFNC. Deaths among patients with HFNC without MV was 11.1%, including 3 LTE´s patients, without differences with those who required low oxygen supplies (8.2%). The patients who received HFNC prior to MV (39%) did not have a different mortality than those required MV initially.
Many factors could had influenced in case fatality rate of these studies, e.g., different populations characteristics, comorbidities prevalence, mix-case severity, treatment protocols, institutional approach heterogeneity and particularly the period under analysis. In our cohort, all hypoxemic cases received oxygen therapy, dexamethasone and anticoagulants [27,31] based on based evidence available at the time of admission as initial therapy. This context may have been a major aspect to benefit patient’s prognosis. Our hospital mortality, global and 28-day, for severe and critical cases is lower than the mentioned national reports but cannot be comparable because of the factors mentioned above.
Roth G, et al. and Docherty A et al. recently published two retrospective series from the first wave in the US (20,736 cases) and UK (63,972 cases) [32,33]. Roth G, et al. [32] reported 15.8%, hospital mortality with a significant reduction from 19.1% between March and April to 10.8% between September and November 2020. In their population, 27% required oxygen therapy, 19,6% were admitted to MV and 34.3% received corticosteroid treatment, with a significant increase throughout the study (22.1% to 68.4%). Authors identified age as largest weight risk factor associated with hospital death, but also male gender, BMI > 45, cancer, stroke, diabetes, and heart failure.
Docherty A, et al. [33], published eligible cases of 247 hospitals in England, Scotland and Wales recruited prospectively in WHO ISARIC cohort protocol, report a significant reduction in hospital mortality at day 28 of 32.3% in March-April to 16.4% in June-August. The authors considered that survival rate improvement, regardless of age, gender, ethnicity, and comorbidities, could be partly explained by case-mix and illness severity, differences in respiratory support (invasive MV (64.1% to 29.4%) and non-invasive MV 22.9% to 47.1%), critical care use, steroid use which could partly reflect accrual of clinical knowledge, as well as other factors, hospital capacity strain, viral inoculum, and community behavior.
Our experience evidenced the feasibility to prepare a single institution to respond to COVID19 pandemic demands with outcomes alike with international reports.
Among the limitations to be observed is that data collection was carried out synchronously with patient care therefore high workload imposed by the pandemic could had led an underreport bias. However, integration of database from the three sources (administrative, mandatory case reporting and clinical history) minimizes the possibility of data loss. Secondly, a significant number of information was obtained from patients self-report or relatives’ interaction so some information biases should be anticipated (e.g.: Hawthorne effect to declare tobacco use in patients with respiratory disease). Thirdly, since it is a private hospital, a selection bias could be postulated due to health coverage. However, daily hospitalizations were allocated by CCC of the Ministry of Health of the Province of Neuquén according to the of beds and resources availability, independently of health coverage and/or third payer. Finally, admitted most patients were diagnosed by direct methods and/or serology considering that sensitivity is 85-95%, it is possible that patients with low or low viral load are underrepresented.
Systematic recruitment of all cases admitted stands out, as well as the prospective collection of data using the mandatory COVID-19 notification form as a basis are two mayor strengths of this protocol. Also, CCC´s patient management and assignment within Province of Neuquén´s hospitals and institutional approach, from diagnosis, outpatient care, face-to-face or by telemedicine, until discharge, prevented to suffer patient lost to follow up.
In conclusion, in our experience, age, reported comorbidities and high oxygen requirements were associated with hospital mortality. Comorbidities and the extension of lung involvement by imaging also provided clues in patients follow-up.
Hospital and 28-day mortality observed in unvaccinated patients hospitalized during the first wave of COVID-19 at the Clínica Pasteur in Neuquén was lower than reports for the same period in Argentina. Case mix and illness severity as well as other factors could had influenced this observation, e.g., early implementation of a contingency plan to ensure institutional readiness, active participation at all levels and processes supervised by institutional leadership, clinical accrual by training and practice, integration of health care assistance teams, and evolving standard of care based on the best scientific evidence available along first wave.
COVID19 in-hospital mortality remain high in comparison with other community acquired respiratory infections, particularly in unvaccinated population and patients receiving invasive mechanical ventilation therefore should be a priority in ongoing research.
References
1. Gao Q, Hu Y, Dai Z, et al. The epidemiological characteristics of 2019 novel coronavirus diseases (COVID-19) in Jingmen, Hubei, China. Med (United States). 2020;99(23):113-122. doi:10.1097/MD.0000000000020605
2. Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA - J Am Med Assoc. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
3. Grasselli G, Pesenti A, Cecconi M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA. 2020;323(16):1545-1546. doi:10.1001/jama.2020.4031
4. Grasselli G, Greco M, Zanella A, et al. Risk Factors Associated with Mortality among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345-1355. doi:10.1001/jamainternmed.2020.3539
5. Redondo-Bravo L, Moros MJS, Sanchez EVM, et al. The first wave of the COVID-19 pandemic in Spain: Characterisation of cases and risk factors for sever. Eurosurveillance. 2020;25(50):1-13.
6. Chhibber-Goel J, Malhotra S, Krishnan NMA, et al. The profiles of first and second SARS-CoV-2 waves in the top ten COVID-19 affected countries. J Glob Heal Reports. 2021:1-7. doi:10.29392/001c.27143
7. World Health Organization W. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Geneva, Switzerland; 2020.
8. Argenzian MG, Bruc SL, Slate CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ. 2020;369(March). doi:10.1136/bmj.m1996
9. Lamfre L, Caro P, Hasdeu S, et al. Modelo de Proyección de Casos Esperados COVID-19 En Argentina 2020. Neuquen; 2020. https://1331c255-c873-472c-8edd-6738111a6c38.filesusr.com/ugd/d623b6_007bc0ad0898495882e61367e1d2644d.pdf.
10. Finelli L, Gupta V, Petigara T, et al. Mortality Among US Patients Hospitalized With SARS-CoV-2 Infection in 2020. JAMA Netw Open. 2021;4(4):e216556-e216556. doi:10.1001/jamanetworkopen.2021.6556
11. Oppenheim B, Gallivan M, Madhav NK, et al. Assessing global preparedness for the next pandemic: Development and application of an Epidemic Preparedness Index. BMJ Glob Heal. 2019;4(1). doi:10.1136/bmjgh-2018-001157
12. Sun Q, Qiu H, Huang M, et al. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):2-5. doi:10.1186/s13613-020-00650-2
13. Manoukian D, Elder M. Mortalidad Por Covid-19 Y Sinergia Con Enfermedades Crónicas Coexistentes En La Provincia Del Neuquén. Rev argentina salud publica. 2021;13(Supl COVID19):e32.
14. National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/. Published 2020. Accessed May 25, 2020.
15. Veltri I, Zabert G, Pelaez J, et al. Plan Institucional COVID19. Clinica Pasteur. Neuquen; 2020.
16. Zabert G, Veltri I, Goya F, et al. Protocolo Tratamiento COVID19 Clinica Pasteur v 1.1. Neuquen; 2020.
17. Zabert G, Veltri I, Goya F, et al. Protocolo Tratamiento COVID19 Clinica Pasteur v 2. Neuquen; 2021.
18. Espinosa L, Ambort C, Vera M, et al. Documento de Comité Asesor de Ética de La Clínica Pasteur Para La Implementación Del Documento de Aspectos Éticos En La Pandemia de COVID19 En El Escenario de Recursos Escasos. Neuquen; 2020. https://drive.google.com/drive/u/0/folders/1BK1rSlpCR2KzydRxRp6KKUsqLezIKsAi.
19. Rosenbaum L. Facing Covid-19 in Italy — Ethics, Logistics, and Therapeutics on the Epidemic’s Front Line. N Engl J Med. 2020:1-3. doi:10.1056/nejmp2005492
20. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N Engl J Med. 2020:1-7. doi:10.1056/nejmsb2005114
21. MSal Neuquen. ASPECTOS ÉTICOS EN LA ATENCIÓN DE LAS PERSONAS DURANTE LA PANDEMIA POR CORONAVIRUS ( SARS-CoV-2 ). Neuquen; 2020.
22. Schönfeld D, Arias S, Bossio JC, et al. Clinical presentation and outcomes of the first patients with COVID-19 in Argentina: Results of 207079 cases from a national database. PLoS One. 2021;16(2):e0246793. doi:10.1371/journal.pone.0246793
23. Boietti BR, Mirofsky M, Valentini R, et al. Análisis Descriptivo De 4776 Pacientes Internados En Servicios De Clínica Médica Por Covid-19. Resultados Del Registro Multicéntrico Argentino - Rema-Covid-19. Medicina (B Aires). 2021.
24. Estenssoro E, Loudet CI, Ríos FG, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. July 2021. doi:10.1016/S2213-2600(21)00229-0
25. Molini W, Gonzalez R, Villalba L, et al. Terapia Nasal De Alto Flujo En Insuficiencia Respiratoria Grave Por SARS-CoV2. Med. 2021;81(21):2-28.
26. Secretaría de Gobierno de Slalud. 4o Encuesta nacional de Factores de riesgo. Principales Resultados. 2019:1-20.
27. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Vol 46. Springer Berlin Heidelberg; 2020. doi:10.1007/s00134-020-06022-5
28. Barrot L, Asfar P, Mauny F, et al. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med. 2020;382(11):999-1008. doi:10.1056/nejmoa1916431
29. Horby P, Lim W. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. 2020:1-11. doi:10.1056/nejmoa2021436
30. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094-1099. doi:10.1111/jth.14817
31. Nadkarni G, Lala A, Bagiella E, et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J Am Coll Cardiol. 2020;76:1815-1826. doi:doi:10.1016/j.jacc.2020.08.041
32. Roth GA, Emmons-Bell S, Alger HM, et al. Trends in Patient Characteristics and COVID-19 In-Hospital Mortality in the United States during the COVID-19 Pandemic. JAMA Netw Open. 2021;4(5):1-9. doi:10.1001/jamanetworkopen.2021.8828
33. Docherty AB, Mulholland RH, Lone NI, et al. Changes in in-hospital mortality in the first wave of COVID-19: a multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9(7):773-785. doi:10.1016/S2213-2600(21)00175-2
Received: February 02, 2022;
Accepted: February 21, 2022;
Published: February 24, 2022.
To cite this article : Gustavo Z, Ignacio V, Ignacio Z, et al. Mortality in Hospitalized Covid19 Patients during First Wave in a Tertiary Hospital in Northern Patagonia. European Journal of Respiratory Medicine. 2022; 4(2): 285 - 293. doi: 10.31488/ EJRM.128.
© 2022 Gustavo Z, et al.
