Research article/ Open Access
DOI:10.31488/EJRM.152
Nebulized Ivermectin to Reduce Active Viral Replication in Patients with Mild to Moderate Covid-19: A Double-Blind, Randomized, Placebo-Controlled Trial
Carlos A. Riveros, Angela Olier Pino, Geoffrey Ling, Miguel Mantilla, Lina F Reyes, Claudia Tejera, Nicole Draghic
*Corresponding author: : Angela Olier Pino; Email: angelaopinoi@gmail.com
Introduction
Since March 2020, the coronavirus pandemic (COVID-19) encouraged the scientific community to introduce multiple pharmacological and non-pharmacological interventions to prevent the global spread of the severe acute respiratory syndrome coronavirus (SARS-CoV-2). The mRNA and vector- based vaccines are of particular importance among all interventions. Vaccine efficacy has been validated in real-world studies, to reduce mortality and disease severity [39,40,41] but, still could be a need of more medications able to reduce viral replication in sick people and prevention of transmission [1,2] especially with new variants on the rise [3].
Most of community infections with SARS-CoV-2 occurs in the initial days when the virus is in its active replication phase [3,4]. Scientific evidence shows that there is no difference in the virus RT-PCR titters in the nasopharynx and upper respiratory tracts in the early infection days among infected patients [8]. Consequently, the use of anti-viral drugs among all affected individuals, that can arrest viral replication in the early stages would be a reasonable way to effectively reduce transmission in the community.
Since subgenomic mRNA is an intermediate product to synthesize and assemble crucial antigenic structural viral proteins like the spike and nucleocapsid, during the replication, this (subgenomic mRNA), could be a potential biomarker to consider to quantify viral replication [4]. Under this concept, determining the amount of sub-genomic mRNA specific to these antigenic proteins can potentially be explored as a sensitive test to assess the real time active viral replication of a patient affected with SARS-CoV-2, even though genomic RNA detection through RT-PCR remains the gold standard assay to detect an infected patient [5-7].
Given the public health emergency during the COVID-19 pandemic, and the risk of new ones to appear, one of the foci has been to repurpose existing drugs to assess their antiviral properties, considering the time and costs associated with research and development of innovative drugs. One such repurposed drug candidate is Ivermectin. It is approved by the US Food & Drug Administration (US FDA) to be used traditionally as an anti-parasitic medication, but it has been shown to inhibit viral replication in in-vitro models [1-9]. Ivermectin has been shown to reduce the SARS-CoV-2 viral load to almost zero within 48 hours in Vero Cell cultures. (1) Moreover, anti-viral efficacy of Ivermectin has been evidenced in in-vitro studies to inhibit the replication of other viruses like the Yellow fever, the Human Immunodeficiency Virus (HIV) and Dengue virus, in in-vitro studies [10]. Additionally, it has also been studied to limit infection caused by the West Nile Virus, Influenza Virus and the Venezuelan equine encephalitis virus [11].
However, there have been ambiguous results when Ivermectin used has been used orally to reduce hospitalization and disease severity in patients with mild to moderate COVID-19. Some trials have tested oral Ivermectin in patients with mild to moderate COVID-19 [1,9,12-15]. that failed to demonstrate to reduce hospitalizations or adverse outcomes
Important studies have shown that the minimum inhibitory concentration for Ivermectin cannot be achieved without using oral doses as high as 100X the recommended dosing for humans, [1,16]. Severe inflammation in the lungs owing to conditions like severe COVID-19 have been shown to further reduce their pulmonary bioavailability making oral doses of Ivermectin less efficient when administered orally. Its dose-limiting adverse effects in patients with severe disease, especially neurotoxicity and possible adverse reactions when co-administered with other drugs like CYP3A4 inhibitors, also make oral Ivermectin a less preferred candidate to reduce viral replication in the respiratory tract and community spread [16]. Orally administered Ivermectin has not shown to be effective to treat or prevent COVID19 [42-45].
The strong anti-viral potential of Ivermectin called our attention to explore a different formulation that allows a more localized spread for the drug to increase its efficacy against SARS-CoV-2 given the ambiguity concerning the pharmacokinetics and bioavailability of oral Ivermectin [16-18].
Previous trials have highlighted the use of Ivermectin using different methods of administration, namely nasal sprays [19], inhaled [20] and nebulized formulations. Specifically, the nebulized formulations have been studied for their safety and efficacy in animal models [21]. Inhaled and nebulized formulations of Ivermectin has shown some results in reducing viral replication, with mild adverse effects and better pharmacokinetics in this setting [19-21].
Considering the safety and novelty of nebulized Ivermectin in mind, we conducted a double-blind, randomized study with nebulized 1% Ivermectin mix with 0.3% Dexamethasone in a dilution rate of 10:1, to patients with mild-to-moderate SARS- CoV-2 infection, a first-of-its-kind human study to demonstrate a reduction in the viral replication
Materials and Methods
Study design and Participants
This randomized, double-blind, placebo-controlled study was conducted between April 3, 2021, to July 8, 2021, by the CIMEDICAL Research Center in Barranquilla, Colombia. The participants were identified from the community as adults who tested positive for SARS-CoV-2 results from RT-PCR tests conducted on nasopharyngeal swab samples at multiple outpatient sites as per information provided by the city IMO clinical laboratory organization Barranquilla, these persons were invited to participate in the study.
Of those identified patients, we applied the following the study design inclusion criteria, individuals that were above 18 years of age at the time of recruitment and diagnosed with mild to moderate SARS-CoV-2 (COVID19) symptomatic or asymptomatic; Oxygen Saturation (Sat O2) at rest > 94% in ambient air, without desaturation with ambulation, and a Respiratory Rate (RR) < 20 per minute.
Exclusion criteria applied as follows: less than 18 years of age, decompensated conditions including Diabetes Mellitus (DM), any cardiovascular complication like Acute Heart Disease (AHD), Congestive Heart Failure (CHF), coronary artery disease (CHD), any chronic pulmonary condition like Chronic Obstructive Pulmonary Disease (COPD), chronic kidney disease (CKD), cancer, any form of immunosuppression, and a history of clinical depression or personality disorders; if they had an RR > 20/min, Pulse > 120 bpm, systolic blood pressure < 90 mmHg, pulmonary arterial (PA) diastolic pressure < 60 mmHg, those appearing toxic and distressed, having Sat O2 at rest <93% in ambient air, or desaturation when walking. The study also excluded patients diagnosed with severe SARS-CoV-2 (COVID-19), patients waiting for admission to the ICU, especially those with asthma, a probability of invasive mechanical ventilation and those needing bronchodilator treatment.
Randomization and Testing
Recruited patients were assigned to one of two possible arms, following a strict sequence of inclusion: Group 1 Received nebulized Ivermectin plus Dexamethasone while Group 2 received a placebo (SSN 0.9%). All patient groups were monitored daily by telemedicine. Patients in Groups 1 and 2 underwent nasopharyngeal sampling and RT-PCR & mRNA at day 1, 3, 5, and 7 following treatment interventions.
15 days post intervention, a physical consultation or a remote, telemedicine visit was conducted to follow-up with the patient’s health condition.
Nebulization of Ivermectin Plus Dexamethasone
Patients who were randomly assigned to group 1 received a nebulized 3.3ml dose, 3 times a day for 5 days through a Pacifica Elite Nebulizer with a flow of 8Lpm under a maximum pressure of 35 PSI. A method piston compressor was used to emit the nebulized particles ranging from 0.5-5µ, to reach the upper and lower airways.
The objective was to reach the upper and lower airways surface where the initial phase of the infection takes place, not the lung tissue itself.
Every 3.3ml of nebulized medication contains 30mg of Ivermectin. About 60% of nebulized medications are lost or not inhaled. Hence, in this case only 12mg (40%) of Ivermectin will effectively reach the airways. Considering the approximate dead space in the lungs to be 150cc, the concentration of Ivermectin delivered to the lungs would be 0.08mg/cc (80,000 ng/mL). Considering previous in vitro research mouse models, the IC50 was found to be (2-2.5µM) ~ 1750 ng/mL, which was enough to reduce viral SARS-CoV-2 RNA load by ~5000 fold [11,22,23].
RT-PCR Testing and Sub-Genomic mRNA Analysis
The nasopharyngeal samples from each patient were analysed no more than three days after the appearance of symptoms, if any. They were stored at 2-8°C within 6h of extraction as per standard protocol mentioned previously, [21] in 50µl of eluate, then stored at -20°C for no more than 12h before PCR-based analysis. All samples then were then stored at -70°C and finally discharged as per protocol of INS (Colombian National Institute of Health), by controlled incineration. The samples then underwent RT-PCR (TIB MolBiol/Cobas z480, Roche/F. Hoffmann-La Roche AG, Basel, Switzerland) for analysis of genomic RNA detecting genes encoding the envelope (E), nucleocapsid ( N) , and RdRp in a monoplex scheme for each target.
RNA was extracted from the chosen samples from both groups, using the VN143 Viral RNA Mini Kit (Genolution, South Korea) using a standard protocol, as directed by the manufacturers [24]. The purified RNA was reverse transcribed using Superscript II (Thermo Fisher Scientific, Massachusetts, USA) and a SARS-CoV-2 specific primer (WHSA-29950R: 5′-TCTCCTAAGAAGCTATTAAAAT-3 ′). The complementary DNA obtained was subjected to qPCR (40 cycles at 94°C for 30s, 56°C for 30s, and 72°C for 1.5 min; optimized to amplify small sub-genomic mRNA) and hot-start PCR using AmpliTaq Gold DNA Polymerase (ThermoFisher Scientific) with FAM WHSA-00025F: 5′- CCAACCAACTTTCGATCTCTTGTA-3′ BHQ1 and FAM WHSA-29925R: 5′-
ATGGGGATAGCACTACTAAAATTA-3′ BHQ1 as primers, as described previously [5]. The genes under observation in both cases were the RNA-dependent RNA Polymerase (RdRp), envelope (E) and Nucleocapsid (N) genes. An internal control probe read in the VIC channel for the RNAase P (RdRp) gene (human) was included as quality control of the sample and the extraction process (with a tolerance limit Ct35).
Ethics and regulatory compliance
All participants gave their written consent before commencing this study. This randomized, double- blind, placebo-controlled study was approved by the Research Ethics Committee of Clinica de la Costa LTDA and Invima (Colombian Regulatory Agency). The study was conducted keeping in mind all necessary Good Clinical Practice Guidelines and in accordance with the tenets of the Declaration of Helsinki.
Statistical analysis
All data were analysed using SPSS (SPSS Inc., Delaware /IBM, New York, USA). Quantitative variables were analysed using frequency and Inter-quartile range (IQR).Then a Kolmogorov-Smirnov Test was used to asses normality of the distribution. Parametric testing was preformed when a normal distribution was reported, and non-parametric testing was done in those variables with non-normal distribution. Bivariable analyses were performed with Wilcoxon singed rank test (to compare quantitative variables between the two groups), McNemar-Bowker Test (to analyse the differences between the categorical variables within the groups), and Analysis of variance – ANOVA for parametric testing and Kruskal-Wallis Test for non-parametric testing (to compare the effects of the drug and the placebo in both groups). A p-value of <.005 was considered significant in all cases.
Analysis was made on SPSS
Frequency and mean (DE)/median (IQR) analysis.
Table 1. Baseline characteristics of patients along with IQR
| Variable | Median (IQR) | Frequency (n) |
|---|---|---|
| Age | 41 (17.5) | |
| Weight (kg) | 67 (30.75) | |
| Height (cm) | 168 (14) | |
| BMI* | 24.93 (9,41) | |
| Gender | ||
| Male | 40.68 (24) | |
| Female | 59.32 (35) | |
| Comorbidities | 16.95 (10) | |
| Unprescribed medicines | 42.7 (25) | |
| Acetaminophen | 16.67 (14) | |
| Colchicine | 5.95 (5) | |
| Ivermectin | 9.52 (8) | |
| anti-inflammatory non-steroidal drugs | 11.90 (10) | |
| Antibiotics | 8.33 (7) | |
| Aspirin | 5.95 (5) | |
| Statins | 9.52 (8) | |
| Cough medications | 9.52 (8) | |
| Anti-histamines | 3.57 (3) | |
| Oral steroids | 7.14 (6) | |
| Vitamins | 9.52 (8) | |
| Inhalers | 2.38 (2) |
Kolmogorov-Smirnov analysis (normality test)
H0= data has a normal distribution (accepted if p value is bigger than p=0,005)
Ha= data has a non-normal distribution (accepted if p value is bigger less than p=0,005). Parameters on sub-genómic RNA and PCR had no normal distribution, that is why data was analyzed as non-normal.
Wilcoxon test (to see differences between the two groups for all the numerical variables).
McNemar-Bowker test (to see differences between the two groups for all the categorical variables
ANOVA test (to see differences between the two groups on the results of sub-genomic RNA and PCR, for every day).
Endpoints
The primary endpoint of this study was to assess the reduction in SARS COV-2 viral replication by determining the amount of sub-genomic mRNA in patient samples after administering the investigative drug.
Results
A total of 90 patients were enrolled to assess eligibility, of those 30 were excluded (21 not meeting criteria, 5 declined to participate, and 4 could not participate due to other reasons. 60 patients were randomized out of which 59 made it to the final set of participants (one patient was eventually excluded due to an active asthma diagnosis with concomitant medication), divided into two groups consisting of 30 patients receiving Nebulized IVM + Dexamethasone and 29 patients receiving placebo (Nebulized SSN).
The median (IQR) age of the patients was 41 (17.5) years, with a body mass index (BMI) 24.93 (9.41). A total of 59.32% (n=35) of the patients were female and 40.68% (n=24) were males. 3.4% patients had a history of arterial hypertension, 5.1% had obesity, 3.4% had thyroid issues, with 16.9% patients reporting at least one coexisting medical condition. All patients were in stable conditions throughout the trial.
Non prescribed medication use: A total of 42.7% patients were consuming unprescribed medicines at the time of the study, out of which 16.67% patients received acetaminophen, 5.95% received colchicine, 9.52% received oral ivermectin, 11.90% received anti-inflammatory non-steroidal drugs, 8.33% received antibiotics (azithromycin, clarithromycin, levofloxacin, amoxicillin, and doxycycline), 5.95% received aspirin, 9.52% received statins, 9.52% received cough medications, 3.57% received antihistamines, 7.14% received oral steroids, 9.52% received vitamins, and 2.38% had inhalers.
Nonparametric statistics was used for the analysis of the differences in use of non-prescribed medication between the groups.
No significant differences were found between age and gender of patients comparing the two groups (Table 2). The p-value for age was 0.25 and that for gender was 0.857.
Table 2. Comparisons of baseline characteristics between treatment and control groups.
| Variable | Treatment group (n=30) | Placebo group (n=29) | ||
|---|---|---|---|---|
| Median (IQR) | Frequency (n) | Median (IQR) | Frequency (n) | |
| Age | 40 (21) | 40 (13) | ||
| Weight (kg) | 76 (28) | 59 (3) | ||
| Height (cm) | 169 (11) | 163 (3) | ||
| BMI* | 27 (9) | 22 (0) | ||
| Gender | ||||
| Male | 63,33 (19) | 55,17 (16) | ||
| Female | 36,67 (11) | 44,82 (13) | ||
Nasopharyngeal samples from all patients were analysed for COVID-19 through RT-PCR for genomic RNA and sub-genomic mRNA levels wherein 100% of these samples were confirmed to be positive for the infection on day 1. Further analysis by RT-PCR and sub genomic mRNA to all of the patients, on days 3, 5 and 7 where preformed. Results upon different genes analysed by RT-PCR and sub genomic mRNA are reported on Table 3.
Table 3. RT-PCR results for CT values of patient samples on days 1,3,5,7 of the intervention.
| Day 1 Median (IQR) | Day 3 Median (IQR) | Day 5 Median (IQR) | Day 7 Median (IQR) | ||
|---|---|---|---|---|---|
| RT- PCR | RNA-dependent | 16.4 | 22.15 | 25.8 | 28.92 |
| RNA polymerase (RdRp gene) | 18.73 | 24.75 | 28.46 | 31.14 | |
| Nucleocapsid(N gene) | 15.54 | 21.71 | 25.07 | 28.02 | |
| Envelope (E gene) | 17.14 | 24.07 | 28.04 | 28.02 | |
| Average | 21.31 | 24.97 | 29.09 | 33.95 | |
| sub genomi c mRNA | cycle threshold (CT) | 7.88 | 6.15 | 4.21 | 1.91 |
| Logarithmic value | 75978980 | 1423472.5 | |||
| Squared value (SQ) | 1 | 4 | 16045.7 | 80.81 |
Alongside, there were no clinical markers showing severe disease in any of the samples collected within the first 5 days of the disease (Table 4).
Table 4. Analysis of markers for severe/critical disease.
| Variable | Median (IQR) | Laboratory Cut-offs for severe disease |
|---|---|---|
| Troponine | 3.95 (2.15) | <14pg/ml |
| PCR | 4.10 (12.18) | <34.67 mg/l |
| D Dimer | 279.50 (492.75) | 0.4 µg/mL or <500 |
| Ferritin | 185.70 (295.38) | <150ng/ml |
| Laboratory results | ||
| VSG(Erythrocyte Sedimentation Rate) | 23 (17.5) | <25 |
| Total bilirubin | 0.33 (0.23) | 1.2 mg/dl |
| Direct bilirubin | 0.14 (0.10) | <0.3 mg/dl |
| Indirect bilirubin | 0.17 (0.11) | 0.2-0.8 mg/dl |
| Aspartate aminotransferase | 23.35 (13.33) | 30 |
| Alanine aminotransferase | 25.95 (16.80) | 30 |
Graphs to compare symptoms among patients treated with Ivermectin + Dexamethasone Vs Placebo days 0 to 5 respectively.
Graphic #1 shows the percentage of patients identified in both groups, medicated with Nebulized Ivermectin + Dexamethasone (Blue color bars), and placebo (Red color), specified by symptoms at day cero(0). The most frequent symptoms reported are: Nasal congestion reported by around 8% of patients in both groups; General Malaise reported by 7.8% og patients in the medication group Vs 5% of patients in the placebo group; Cough was reported in 6.7% in both intervention and placebo groups and Fever reported in 2.2% of the intervention medication group and 3.9% of the placebo group. Other symptoms reported include Back pain reported by 3.3% of the intervention medication patients and 1.1% of placebo group. Other less reported symptoms at day cero(0) are sore throat and Chest pain.
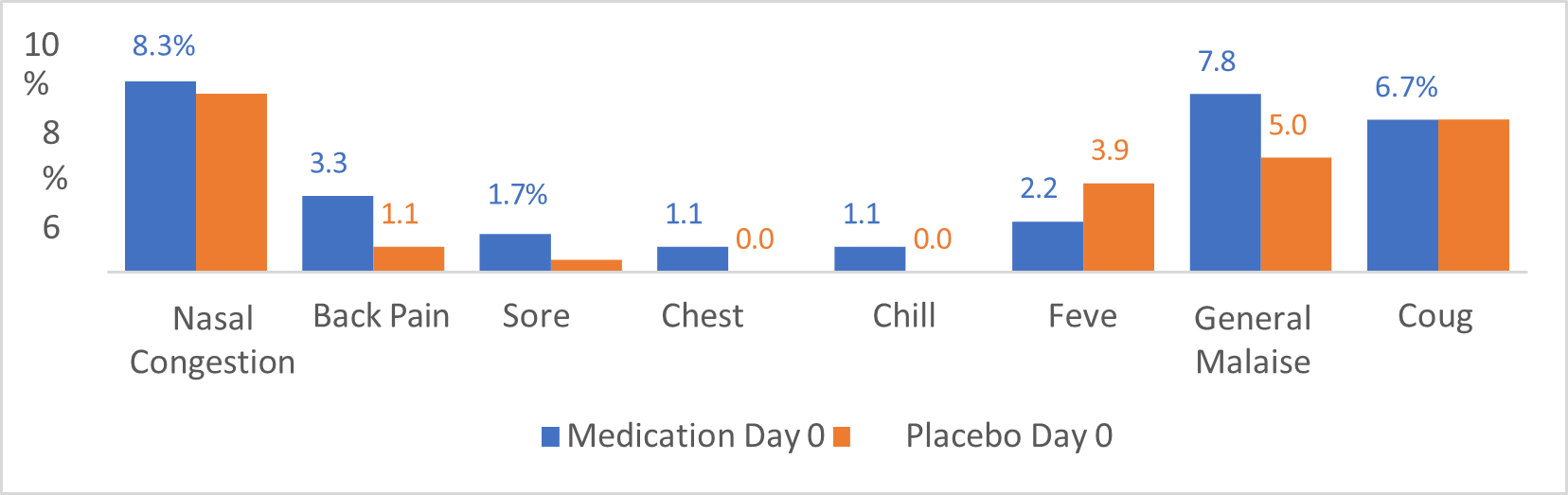
Graphic 1:shows the percentage of patients identified in both groups, medicated with Nebulized Ivermectin + Dexamethasone (Blue color bars), and placebo (Red color), specified by symptoms at day cero(0).
Graphic #2 illustrates the percentage of patients identified in both groups, medicated with Ivermectin + Dexamethasone (Blue color bars), and placebo (Red color bars), specified by symptoms at day 5 (last day of the treatment). As shown in graphic #2, the symptoms reported during day o, were also followed at day 5.Nasal congestion showed important reduction in both groups, medication intervention (Blue bars) and placebo (red bars) from approximately 8% in day Cero(0), (graphic #1), to 0.9% in day 5 (ghraphic #2). The General Malaise was reported significantly less frequent by day 5 (2.6%) in the intervention group (blue bar), from 7.8% reported during day 0, showing also a significant reduction compared to placebo group 5% during day 0 to 5.1% during day 5. The cough was reported in 5.1% of medication patients (Blue bar), on day five compared to 6.7% of patients reporting this symptom at day 0, additionally, this same symptom was reported in the placebo group(Red bar), by 6.8% of patients at day 5 compared to 6.7% during day 0.
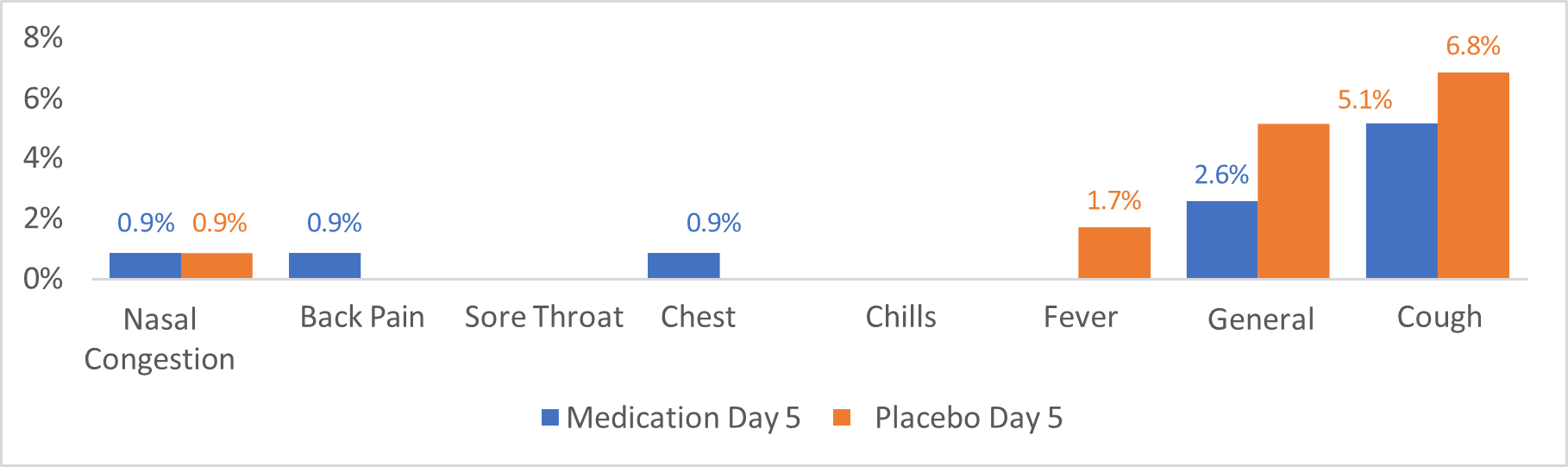
Graphic 2:illustrates the percentage of patients identified in both groups, medicated with Ivermectin + Dexamethasone (Blue color bars), and placebo (Red color bars), specified by symptoms at day 5 (last day of the treatment). As shown in graphic #2, the symptoms reported during day o, were also followed at day
In general, the patients in the intervention (Ivermectine + Dexamethasone) group, reported less symptoms after 3-5 days of treatment compared to placebo goup.
Figure 1. As the treatment progressed, there is a steady and significant reduction in the sub- genomic mRNA on a day-wise basis (Figure 1).
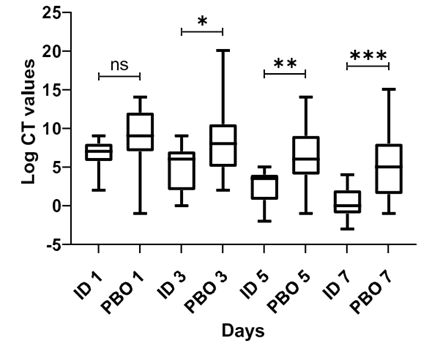
Figure 1:Sub-genomic mRNA count on a day-wise basis. CT: cycle threshold, ID: intervention drug, PBO: placebo, ns: non-statistical significance (p=0,23), *p=0,002, **p<0,00001, ***p<0,00001 .Figure 1. As the treatment progressed, there is a steady and significant reduction in the sub- genomic mRNA on a day-wise basis (Figure 1).
This reduction became increasingly statistically significant, as the treatment progressed (Table 5). The data from these variables was analysed by non-parametric statistics (Kruskal-Wallis Test), considering the non-normal distribution of the data
Table 5. Reduction in Sub-genomic mRNA CT value in days 1-3-5-7.
| Variable | Treatment group (n=30) Median | Placebo group (n=29) Median | P value |
|---|---|---|---|
| Logarithmic CT values (day 1) | 7 | 9 | 0.23 |
| Logarithmic CT values (day 3) | 6 | 8 | 0,002 |
| Logarithmic CT values (day 5) | 4 | 6 | <0.00001 |
| genomic Logarithmic CT values (day RNA 7) | 0 | 5 | <0.00001 |
Graphics 2 and 3 show the reduction of Sub genomic mRNA quantifications already explained in figure2. As previously explained, there is a statistically significant reduction in Sub genomic mRNA in the IVM+ Dexamethasone group from day 0 to day 7 when compared to the placebo group.
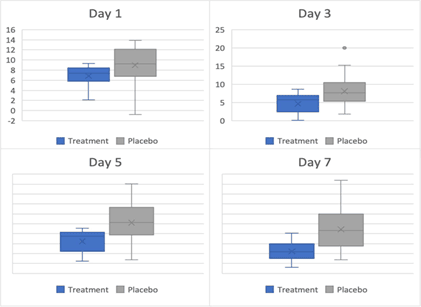
Figure 2:Box and whiskers plot for sub-genomic mRNA on days 1,3,5 and 7 respectively. Figure 2 shows a progressive reduction of Sub-genomic mRNA measured at days 1, 3, 5 and 7.
Table 6. RT-PCR results comparing the p-values for different genomic RNA across days 1,3,5 and 7 (all logarithmic values).
| Gene RdRp: Treatment | Placebo | p-value | |
|---|---|---|---|
| Day 1 | 16 | 17 | 0.39 |
| Day 3 | 20 | 25 | 0.97 |
| Day 5 | 24 | 27 | 0.93 |
| Day 7 | 28 | 29 | 0.42 |
| Gene N: Treatment | Placebo | p-value | |
| Day 1 | 19 | 18 | 0.34 |
| Day 3 | 22 | 27 | 0.15 |
| Day 5 | 23 | 29 | 0.2 |
| Day 7 | 30 | 34 | 0.71 |
| Gene N: Treatment | Placebo | p-value | |
| Day 1 | 16 | 15 | 0.2 |
| Day 3 | 19 | 23 | 0.62 |
| Day 5 | 23 | 26 | 0.52 |
| Day 7 | 27 | 30 | 0.43 |
| Average Treatment | Placebo | p-value | |
| Day 1 | 17 | 17 | 0.61 |
| Day 3 | 21 | 25 | 0.55 |
| Day 5 | 25 | 28 | 0.49 |
| Day 7 | 32 | 35 | 0.77 |
The presence not prescribed medication was analysed for any possible bias, no statistical significance was demonstrated between the use of non-prescribed medications and the intervention in groups (Table 7).
Table 7.Patients taking over-the-counter medication, not prescribed by the physician, during the study.
| Variable | Treatment group (n=30) | Placebo group (n=29) |
|---|---|---|
| Median Frequency (IQR) (n) | Median Frequency (IQR) (n) | |
| Unprescribed medicines Acetaminophen | 15.62(10) | 30 (3) |
| Colchicine | 6.25 (4) | |
| Ivermectin | 12.5(8) | 10 (1) |
| anti-inflammatory non-steroidal drugs | 15.62(10) | |
| Antibiotics | 12.5(8) | 30 (3) |
| Aspirin | 6.25 (4) | |
| Statins | 3.12 (2) | |
| Cough medications | 3.12 (2) | 30 (3) |
| Anti-histamines | 4.68 (3) | |
| Oral steroids | 9.38 (6) | |
| Vitamins | 6.25 (4) | |
| Inhalers | 4.68 (3) |
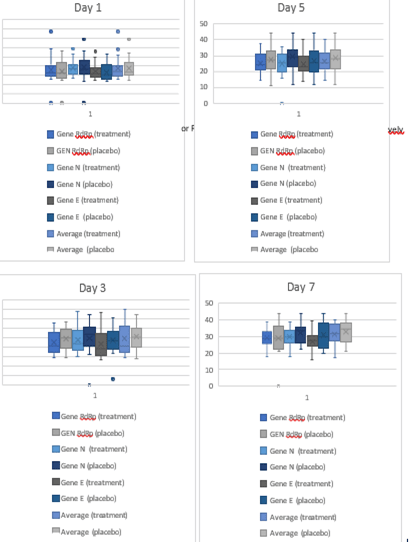
Figure 3:Box and whiskers plot for sub-genomic PCR data observed on days 1,3,5 and 7 respectively.
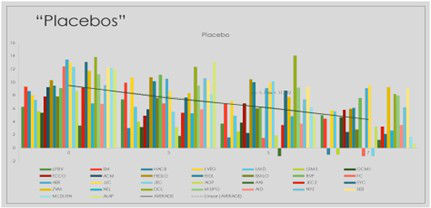
Graphic 3:Distribution of Sub genomic Messenger RNA among patients randomized to placebo group quantified on days 0-3-5-7 of the trial.
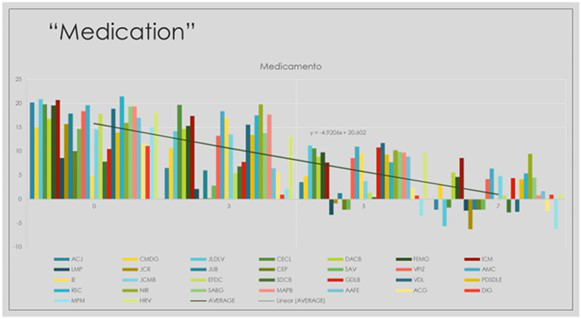
Graphic 4:Distribution of Sub genomic Messenger RNA among patients randomized to IVM+- Dexamethasone group quantified on days 0-3-5-7 of the trial.
Discussion
We considered the availability, cost-effectiveness and proven anti-viral potential of Ivermectin in previous in-vitro studies not only with SARS COV-2, but also for yellow fever virus, the human immunodeficiency virus (HIV), dengue virus, West Nile Virus, influenza Virus and the Venezuelan equine encephalitis virus to conduct this double blind trial [10,11,26]. A 3 ml dose of nebulized Ivermectin mixed with Dexamethasone in a 10:1 ratio was randomized along with a placebo among 59 patients with mild to moderate symptoms and a confirmed PCR result, positive for SARS-CoV-2, thrice daily for 5 days, in an attempt to evaluate its efficacy in reducing viral replication. The quantification of the sub-genomic mRNA was used in this trial to measure the viral replication.
We found a statistic significant reduction in the count of sub-genomic mRNA in the samples taken from sick patients receiving nebulized Ivermectin compared blindly with placebo at day 7 (P Value <0.05). This was the first human based study to evaluate nebulized Ivermectin as a potential medication to be explored to reduce viral replication of SARS-COV2 when used via nebulization directly in the airways. Additionally, this study highlights the potential use of viral sub-genomic messenger RNA analysis to evaluate the active replication of an RNA virus a strategy which could be useful for other RNA viruses as well [11,23].
The most common route of administration for Ivermectin in different studies thus far has been the oral route. However, different concentrations of oral dosing have been unsuccessful in reaching the required inhibitory concentrations in the lungs (IC50) which can be attributed to the molecule’s high lipophilicity, its low ionization at physiologic pH [23].The high crystal lattice energy of Ivermectin as a molecule, makes it is less soluble in aqueous solution and more soluble in amorphous forms, as suggested by Mansour et al. [16].
As Schmith et al. showed in his study, the approved dose of oral Ivermectin, (200 µg/kg) once weekly was not sufficient in reaching the IC50 even after increasing the dosage to 60mg every 3 days or 120mg once weekly, which should at best approach the inhibitory concentration to 1/5th of the recommended amount (2µM) in the lungs [11,23]. Moreover, Jermain et al. developed a simulation of Ivermectin's exposure to the lungs through oral routes in physiologically-based pharmacokinetic models at doses of 12, 30, and 120 mg. Despite of this, the maximum concentration achieved in the lungs was 772 ng/mL, lower than the reported IC50 for ivermectin in vitro (1750 ng/mL) [27].
Ivermectin showed to reduce SARS-CoV-2 viral load by ~5000 fold within 48 hours in Vero Cells (In Vitro), which has led to [27] clinical trials dedicated to finding the optimal dosing for Ivermectin against COVID-19, not only evaluating Ivermectin’s potential in treating COVID-19 but also its prophylactic potential [11,28].Some of these studies have reported an improvement in overall patient health with significant reduction in mortality [15,28]. However, it still remains to be understood if Ivermectin can eventually reduce in vivo, active viral replication and prevent community spread of COVID-19.
Ivermectin’s safety profile is well established from previous studies. There have been very low rates of incidence of adverse effects, mostly related to the inflammatory response to different infections including itching, rash, swollen lymph nodes, joint pain, fever, malaise and headache [28]. Even with doses as high as 1200 μg/kg for 5 days (highest oral dosage in clinical trials, in the COVER study) showed no signs of severe adverse effects [1].
There have been a few studies using the nasal formulations as an attempt to make more of the compound available freely in the pulmonary airspace. Safety studies conducted on piglets receiving Ivermectin either orally (0.2 mg/kg) or by one or two nasal spray doses showed that there were no systemic adverse effects in the recipients, and that administering the nasal spray every 12 hours, helped the lungs and nasopharyngeal tissues receive a high concentration of Ivermectin which also persisted through the day (in a span of 24 hours). A key observation in this case was that the ratio between the nasal spray/oral concentration in the nasopharyngeal tissue increased significantly from 0.88 (one spray application) to 2.10 (two spray applications), from 0.24 to 0.63 in the lungs and from 0.25 to 0.57 in the plasma [29]. A drawback was that there were no observations made on a regular basis to understand the persistence of the drug in the lungs.
The study by Aref et.al. with 114 human subjects with mild COVID-19 who were randomized to a mucoadhesive nanosuspension spray twice daily along with routine Egyptian protocols for COVID-19 treatment against a placebo and assessed for negative RT-PCR reports and improved clinical parameters for 7 days. The patients receiving the nasal mucoadhesive spray developed clinical parameters closer to normalcy more significantly within 7 days of treatment, with no adverse effects [19]. This study relied on negative RT-PCR results for a confirmed negative diagnosis, and did not assess the contagiousness of the disease due to viral replication.
Chaccour et al. assessed the safety of nebulized Ivermectin by randomizing 14 rats into three target dosing groups, with a lower dose (80–90 mg/kg), a higher dose (110–140 mg/kg) or ethanol vehicle only. They were monitored for 7 days post this intervention, for the levels of the drug in the lungs and plasma as well as any adverse effects and clinical parameters which may hint at any deterioration in health. The study concluded that nebulized Ivermectin was safely sustained in detectable concentrations in the lungs for seven days, with concentrations up to 524.3 ng/g for high-dose male and 27.3 ng/g for low-dose females [21].
Supported in these observations, our study was conducted as a pilot human study to assess the safety as well as efficacy of nebulized Ivermectin to reduce SARS-COV-2 viral replication in patients with mild to moderate COVID-19. Nasopharyngeal samples were chosen for this study since multiple studies have established a strong correlation with an active infection, as well as viral load count [30-32]. Additionally, nasopharyngeal samples are validated by the US FDA and CDC as the gold standard for detecting genomic RNA for COVID-19 [33,34]. Sub-genomic mRNA analysis, although not the gold standard test, has been effectively used in previous trials, for an early diagnosis of COVID-19 patients [35].
The decision to use dexamethasone in combination with Ivermectin was made based in the fact that Ivermectin administered intratracheally (inhaled) in the lungs in doses of 0.2, 0.4 and 0.8 mg/kg had shown to attract more pro-inflammatory cytokines (TNF-α, IL-3, IL-6) with a paralleled reduction in IL- 10, an anti-inflammatory cytokine. Also the Hydroxy propyl-β-cyclodextrin makes Ivermectin more soluble and stable in the inhalable formulation [16].Additionally, the use of Dexamethasone chemically enhances the dissociation of the hydroxyl bonds of Ivermectin and helped in making it suitable for nebulized administration.
Our study also explores the potential use of sub-genomic mRNA analysis. The sub-genomic mRNA is a potential test to count the viable virus during its active transcription and translational modifications for assembly of the structural proteins [4]. On the other hand, rapid antigen tests are indicative of an active infection, with lower sensitivity (~18-50%) but a higher specificity (~100%), irrespective of the cycle threshold (CT) value, as compared to genomic RT-PCR analysis [36]. This also highlights a possible correlation between these two test methodologies. Though this analysis was out of scope for this study, it can be encouraged in future studies.
The results from this non completely conclusive study, encourage future studies and validation of the sub-genomic mRNA as an analytical method to detect active infection by SARS-COV2 and other viruses.
Limitations
We had limitations during this study to be disclosed; first, the ongoing strict lockdown in Colombia by the time of the trial, obstructed the mobilization of Medical Doctors which limited the collection of some clinical data. Secondly, the lack of immunological or other parameters to understand the implications of Dexamethasone. Third, the small size of the sample to lead to definite conclusions. Several questions remain not answered, however our trial could contribute to open the door to think about the potential use of Ivermectin via Nebulized, instead of orally to treat respiratory viral infections including but not limited to COVID 19
Conclusion
This was the first human based trial assessing in vivo, the possible benefits of low cost and low side effect of nebulized Ivermectin in reducing the SARS-COV-2 replication using the Subgenomic Messeger RNA count as a measure of active viral replication in patients positive for COVID-19.
This double-blind randomized trial also highlights the potential use of sub-genomic mRNA tests to determine the quantification of SARS-COV-2 viral replication in vivo. Also, encourage the development of easier sampling and processing methods, to consider further research to validate the use sub- genomic mRNA testing as screen and replication status for COVID-19 and other viral infections in the future.
References
1. Buonfrate D, Chesini F, Martini D, Roncaglioni MC, Ojeda Fernandez ML, Alvisi MF, et al. High- dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial. Int J Antimicrob Agents. 2022 Feb;59(2):106516.
2. Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021 May 13.
3. He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 May 1;26(5):672–5.
4. Kim JY, Bae JY, Bae S, Cha HH, Kwon JS, Suh MH, et al. Diagnostic usefulness of subgenomic RNA detection of viable SARS-CoV-2 in patients with COVID-19. Clin Microbiol Infect. 2022 Jan;28(1):101–6.
5. Perera RAPM, Tso E, Tsang OTY, Tsang DNC, Fung K, Leung YWY, et al. SARS-CoV-2 Virus Culture and Subgenomic RNA for Respiratory Specimens from Patients with Mild Coronavirus Disease. Emerg Infect Dis. 2020 Nov;26(11):2701–4.
6. Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 Jun;20(6):656–7.
7. Than HM, Nong VM, Nguyen CT, Thi Tran NH, Do CD, Pham TN. Management of mild cases of COVID-19 in low-resource countries: An experience in Vietnam. J Microbiol Immunol Infect. 2021 Feb;54(1):139–40.
8. Franco-Paredes C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect Dis. 2022 Jan 1;22(1):16.
9. Popp M, Stegemann M, Metzendorf MI, Gould S, Kranke P, Meybohm P, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev. 2021 Jul 28.
10. Lundberg L, Pinkham C, Baer A, Amaya M, Narayanan A, Wagstaff KM, et al. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res. 2013 Dec;100(3):662–72.
11. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020 Jun 1; 178:104787.
12. Lim SCL, Hor CP, Tay KH, Mat Jelani A, Tan WH, Ker HB, et al. Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial. JAMA Intern Med. 2022 Apr 1;182(4):426–35.
13. Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021 Feb; 103:214–6.
14. Elalfy H, Besheer T, El-Mesery A, El-Gilany A, Soliman MA, Alhawarey A, et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021 Mar 11.
15. Kow CS, Merchant HA, Mustafa ZU, Hasan SS. The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis. Pharmacol Rep. 2021;73(5):1473–9.
16. Mansour S, Shamma R, Ahmed K, Sabry N, Esmat G, Mahmoud A, et al. Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study. Int Immunopharmacol. 2021 Oct; 99:108004.
17. Duthaler U, Suenderhauf C, Karlsson MO, Hussner J, Meyer zu Schwabedissen H, Krähenbühl S, et al. Population pharmacokinetics of oral ivermectin in venous plasma and dried blood spots in healthy volunteers. Br J Clin Pharmacol. 2019 Mar;85(3):626–33.
18. González Canga A, Sahagún Prieto AM, Diez Liébana MJ, Fernández Martínez N, Sierra Vega M, García Vieitez JJ, et al. The Pharmacokinetics and Interactions of Ivermectin in Humans—A Mini- review. AAPS J. 2008 Jan 25;10(1):42–6.
19. Aref ZF, Bazeed SEES, Hassan MH, Hassan AS, Rashad A, Hassan RG, et al. Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19. Int J Nanomedicine. 2021 Jun 15; 16:4063–72.
20. Albariqi AH, Wang Y, Chang RYK, Quan DH, Wang X, Kalfas S, et al. Pharmacokinetics and safety of inhaled ivermectin in mice as a potential COVID-19 treatment. Int J Pharm. 2022 May 10;619:121688.
21. Chaccour C, Abizanda G, Irigoyen-Barrio Á, Casellas A, Aldaz A, Martínez-Galán F, et al. Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose- ranging study in rats. Sci Rep. 2020 Oct 13; 10:17073.
22. Shukla DrAK, Misra DrS. Antiviral Effects of Ivermectin in COVID-19- Clinically Plausible? Int J Infect Dis. 2021 Aug; 109:91.
23. Schmith VD, Zhou J (Jessie), Lohmer LRL. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin Pharmacol Ther. 2020 Oct;108(4):762–5.
24. Genolution Nextractor ® NX-48S Automated DNA/RNA Extractor . Jant pharmacal corporation. 2022 Jun 14.
25. Long S. SARS-CoV-2 Subgenomic RNAs: Characterization, Utility, and Perspectives. Viruses. 2021 Sep 24;13(10):1923.
26. Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am J Ther. 2021 Aug; 28(4):e434.
27. Jermain B, Hanafin PO, Cao Y, Lifschitz A, Lanusse C, Rao GG, et al. Development of a Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung Exposure in Humans Following Oral Administration of Ivermectin for COVID-19 Drug Repurposing. J Pharm Sci. 2020 Dec 1;109(12):3574–8.
28. Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. Am J Ther. 2021 Apr 22;28(3):e299–318.
29. Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model | Elsevier Enhanced Reader.2022 Aug 10.
30. Sharma K, Aggarwala P, Gandhi D, Mathias A, Singh P, Sharma S, et al. Comparative analysis of various clinical specimens in detection of SARS-CoV-2 using rRT-PCR in new and follow up cases of COVID-19 infection: Quest for the best choice. PLOS ONE. 2021 Apr 5;16(4): e0249408.
31. Ünsaler S, Okan A, Tekin S, Hafız AM, Gökler O, Altuntaş O, et al. Comparison of nasopharyngeal swab and nasopharyngeal aspiration in adults for SARS-CoV-2 identification using reverse transcription-polymerase chain reaction. J Med Virol. 2021;93(12):6693–5.
32. Zollo M, Ferrucci V, Izzo B, Quarantelli F, Domenico CD, Comegna M, et al. SARS-CoV-2 Subgenomic N (sgN) Transcripts in Oro-Nasopharyngeal Swabs Correlate with the Highest Viral Load, as Evaluated by Five Different Molecular Methods. Diagnostics. 2021 Feb;11(2):288.
33. Lee RA, Herigon JC, Benedetti A, Pollock NR, Denkinger CM. Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: a Systematic Review and Meta-analysis. J Clin Microbiol. 2021 Apr 20;59(5): e02881-20.
34. Huang L, Zhang X, Zhang L, Xu J, Wei Z, Xu Y, et al. Swab and Sputum SARS-CoV-2 RNA- Negative, CT-Positive, Symptomatic Contacts of COVID-19 Cases: A Hypothesis-Generating Prospective Population-Based Cohort Study of Eight Clusters. Front Med. 2022 Nov 18.
35. Dong X, Penrice-Randal R, Goldswain H, Prince T, Randle N, Donovan-Banfield I, et al. Analysis of SARS-CoV-2 known and novel subgenomic mRNAs in cell culture, animal model, and clinical samples using LeTRS, a bioinformatic tool to identify unique sequence identifiers. GigaScience. 2022 May 26;11: giac045.
36. Wölfl-Duchek M, Bergmann F, Jorda A, Weber M, Müller M, Seitz T, et al. Sensitivity and Specificity of SARS-CoV-2 Rapid Antigen Detection Tests Using Oral, Anterior Nasal, and Nasopharyngeal Swabs: a Diagnostic Accuracy Study. Microbiol Spectr. 10(1): e02029-21.
37. Rao M, Rashid FA, Sabri FSAH, Jamil NN, Zain R, Hashim R, et al. Comparing Nasopharyngeal Swab and Early Morning Saliva for the Identification of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2021 May 1;72(9): e352–6.
38. Higgins TS, Wu AW, Ting JY. SARS-CoV-2 Nasopharyngeal Swab Testing—False-Negative Results From a Pervasive Anatomical Misconception. JAMA Otolaryngol Neck Surg. 2020 Nov 1;146(11):993–4.
39. Eyre. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N Engl J Med. 2022 Feb 24;386(8):744-756
40. Mostaghimi. Prevention of host-to-host transmission by SARS-CoV-2 vaccines. Lancet Infect Dis. 2022 Feb;22(2):e52-e58
41. Martínez-Baz. Effect of COVID-19 vaccination on the SARS-CoV-2 transmission among social and household close contacts: A cohort study. J Infect Public Health. 2023 Mar;16(3):410-417
42. Bramante. COVID-OUT Trial Team. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Engl J Med. 2022 Aug 18;387(7):599-610.
43. Marcolino. Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype. BMC Infect Dis. 2022 Jul 23;22(1):639.
44. Hill. Ivermectin for the prevention of COVID-19: addressing potential bias and medical fraud. J Antimicrob Chemother. 2022 Apr 27;77(5).
45. Roman. Ivermectin for the Treatment of Coronavirus Disease 2019: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin Infect Dis. 2022 Mar 23;74(6):1022- 1029.
Received:January 10, 2025;
Accepted: January 27, 2025;
Published: January 29, 2025.
To cite this article : Riveros CA, Pino AO, Ling G, Mantilla M, Reyes LF, Tejera C, Draghic N. Nebulized Ivermectin to Reduce Active Viral Replication in Patients with Mild to Moderate Covid-19: A Double-Blind, Randomized, Placebo-Controlled Trial. European Journal of Respiratory Medicine. 2025; 7(1): 449-459. doi: 10.31488/EJRM.152.
© The Author(s) 2025. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
