Research article/ Open Access
DOI:10.31488/EJRM.138
Pirfenidone Therapy in Coal Workers’ Pneumoconiosis with Pulmonary Fibrosis and Associated Functional Impairment
Emory H Robinette , Retha Robinette PharmD
1. Pulmonary Research of Abingdon, USA
*Corresponding author: Emory Robinette, Pulmonary Research of Abingdon, 271 East Valley Street, Abingdon, Va. 24210, USA
Abstract
Progressive massive fibrosis in coal miners (CWP/PMF) is an acquired respiratory disorder associated with lung function loss, end stage lung disease and results in severe disability or death. Symptomatic treatment utilizing the standard of care has not changed the decline of lung function or ultimate outcome. Antifibrotic therapy with pirfenidone was re-purposed to determine the impact on lung function in CWP/PMF. This single arm interventional study enrolled 50 coal miners with CWP/PMF at a single center from 2020 to 2022. A control population of miners with CWP/PMF confirmed potential severe decline of lung function over 12 months. Initial screening for study enrollment required miners to have worked at least 15 years in a coal mining environment, have a chest CT scan or chest x-ray consistent with CWP/PMF. Pulmonary function test required an FEV1 of ≤ 75%, or FVC ≤ 80%, or reduction of DLCO ≤ 70%. Miners demonstrated at least a 5% loss in lung function (FEV1, or FVC, or DLCO) within 36 months prior to enrollment. Primary objective for this study was to determine the impact of pirfenidone on rate lung function (FVC) decline in patients with coal workers’ pneumoconiosis with progressive massive fibrosis (CWP/PMF). Secondary objectives included rate of decline of FEV1, DLCO and 6-minute walk test. Chest CT scans were reviewed to determine if radiographic progression occurs during therapy. Results: A total of 31 patients completed at least 12 months of Pirfenidone. Enrolled miners demonstrated primary air flow obstruction with reduction in FEV1. Pirfenidone therapy reduced the rate of decline of FVC, FEV1, and DLCO in CWP/PMF compared to control population. A positive impact of 6-minute walking distance was observed. SGRQ scores were stable during antifibrotic therapy (P< 0.05) compared to baseline during trial, but dyspnea index increased following discontinuation of antifibrotic therapy. Serial CT chest during study failed to demonstrate significant radiographic progression.
Introduction
Treatment of CWP/PMF has been symptomatic and those with end-stage disease are candidates for lung transplantation. Because CWP/PMF cannot be cured, reduction of dust inhalation was the only alternative to slow pulmonary fibrosis progression and associated loss of lung function. Individuals develop occupational lung disease with associated pulmonary fibrosis and fibrotic reaction with coalescent nodules throughout the lung zones. Pirfenidone (PFD) has been approved for the treatment of pulmonary fibrosis, but the mechanism of action has not been fully explained. It has been proposed the fibrotic process in IPF patients limit the activation and differentiation of fibroblast into myofibroblast. Miners with CWP/PMF were enrolled in a clinical trial examining pirfenidone administration for fibrotic related lung disease. Primary objective for this study was to determine the impact of pirfenidone on rate lung function (FVC) decline in patients with coal workers’ pneumoconiosis with progressive massive fibrosis (CWP/PMF). Coal mine dust causes a spectrum of lung disease collectively termed coal mine dust lung disease (CMDLD). These include interstitial lung disease, silicosis, mix dust pneumoconiosis, dust related diffuse fibrosis, and chronic obstructive pulmonary disease [9]. Secondary objectives included rate of decline of FEV1, DLCO and 6-minute walk test with SGRQ scores. Chest CT scans were reviewed to determine if radiographic progression occurred during therapy.
Study Design and Methods
This single arm interventional study enrolled 50 coal miners with CWP/PMF at a single center from 2020 to 2022. To determine the potential impact of antifibrotic therapy, 40 CWP/PMF patients (control population from our clinical practice 2015-2018) were reviewed to understand the disease progression as a comparison. Selected patients demonstrated a severe decline of lung function over 12 months. Calculating average decline, the FVC decreased by 330 mL, FEV1 decreased by 300 mL and 7.5% decline in DLCO in 12 months. Control patients and enrolled patients were selected from clinical pulmonary practice. Initial screening for study enrollment required miners to have worked at least 15 years in a coal mining environment, have a chest CT scan or chest x-ray consistent with CWP/PMF. Pulmonary function test required an FEV1 of ≤ 75%, or FVC ≤ 80%, or reduction of DLCO ≤ 70%. Miners demonstrated at least a 5% loss in lung function (FEV1, or FVC, or DLCO) within 36 months prior to enrollment. Primary objective for this study was to determine the impact of pirfenidone on rate lung function (FVC) decline in patients with coal workers’ pneumoconiosis with progressive massive fibrosis (CWP/PMF). Secondary objectives included rate of decline of FEV1, DLCO and 6-minute walk test. Chest CT scans were reviewed to determine if radiographic progression occurs during therapy. Pulmonary Function test were performed using a hospital-based Vmax Encore 22 Pulmonary Function Analysis Machine. All participants signed an informed consent Advarra IRB approved as phase 2 study on 6/22/2020.
At study enrollment, miners demonstrated an obstructive lung defect more than restriction as a functional impairment. A total of 31 patients completed at least 12 months of Pirfenidone. Average age completing study was 65.3 years. 18 of 31 were nonsmokers with average pk/year of 9.3 in former smoking participants. Pulmonary function studies were performed every 6 months with spirometry, lung volumes, and diffusion capacity. Study measurements were performed at baseline (0), 6 month (6M), 12 months (12M), 18 months (18M) and 3 months post last dose (3MP). PFTs were compared to baseline assessment for change in FVC or FEV1 or DLCO. SGRQ scores with exertion and dyspnea index were additionally collected.
Patient Reported Outcomes Quality of life questionnaire (SGRQ), diaries documenting medical problems/medications taken, health care resource utilization and missed study medication were reviewed at each visit with appropriate documentation in patients visit. SGRQ scores were correlated with other measures of disease activity such as cough, dyspnea, 6MWT and FEV1.
3-month recall tool was used to provide the health status/health-related quality of life measurements in this study. A total score combining each domain was calculated. In each case the lowest possible value is zero and the highest is 100. Higher values correspond to greater impairment of quality of life. 6 Minute Walk Test was performed every 6 months to include oxygen saturation study and documentation of distance walked within 6 minutes. A standard treadmill protocol was utilized, and Borg scale recorded with comparison to baseline dyspnea and exertion fatigue. Screening studies included complete blood count with differential, complete metabolic panel including safety liver and renal function test. All safety laboratory studies were performed per package insert, including monthly liver function testing for the first six months Smoking status/cessation counseling were recorded at each visit. Continuous variables were compared using student t-test and regression analysis confirmed correlation of all collected data. All data are presented as mean ± sem. Statistical analyses were applied using t-tests and one-way ANOVA to determine statistical significance. A two-sided p-value of <0.05 is considered as statistically significant.
Results
During this study, a total of 31 patients completed at least 12 months of Pirfenidone. Enrolled miners demonstrated primary air flow obstruction with reduction in FEV1. Baseline FEV1 was 1.71 L ± 0.57 (53% ± 0.16%). After 6 months of therapy the FEV1 was 1.70L ± 0.17 (54% ± 0.18%) Following 12 months of therapy, FEV1 was 1.67L ± 0.58 (54% ± 0.17%). Individuals completing 18 months of therapy FEV1 was 1.68L ± 0.62 (54% ± 0.18%). Following discontinuation of therapy at 3 months, FEV1 was 1.68L ± 0.62 (53% ± 0.16%) Predicted FEV1 at 18M was 1.61L. There was no significant decline in FEV1 (P<0.05) during therapy (Figure 1).
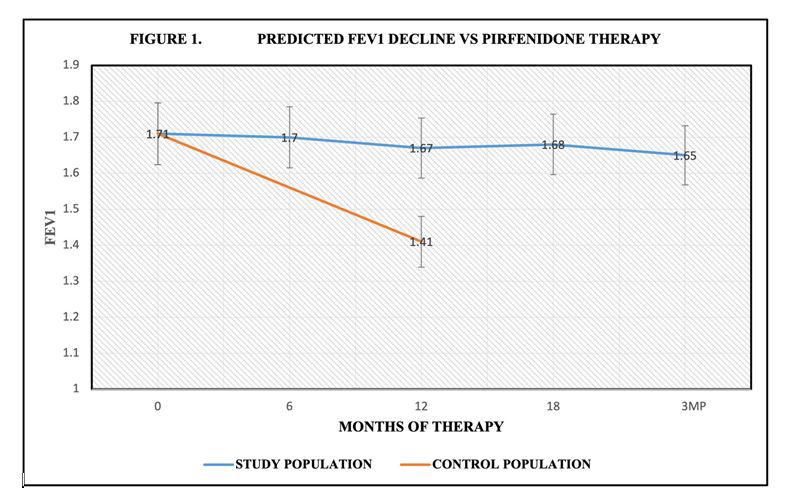
Figure 1:Pirfenidone reduced rate of decline in FEV1during 18 months of therapy compared to control population. Control CWP/PMF received standard care in pulmonary practice.
Baseline FVC average was 3.15L ± 0.75 (74% ± 0.17%) At 6 months of therapy, the FVC was 3.14 L± 0.75 (75% ± 0.17). Following 12 months of therapy, the FVC was 3.05L ± 0.77 (74% ± 0.17%), At 18 months the FVC was 3.10L ± .80 (74% ±0.17%). 3-month post drug discontinuation, the FVC was 3.07L ± .77 (73% ± 0.16%). Control population FVC @ 12 months was 2.80L (P< 0.05) for FVC during trial (Figure 2).
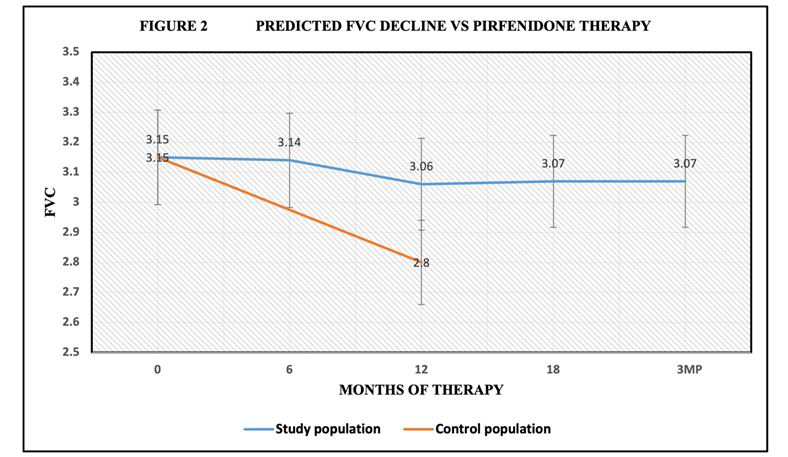
Figure 2:Pirfenidone reduced rate of FVC decline during 18 months of therapy compared to control population. Control CWP/PMF received standard care in pulmonary practice.
Baseline mean DLCO was 72.1% ± 5.6%, at 6 months DLCO was 72.4% ± 16.2%. Following 12 months of therapy the DLCO was 67.5% ± 16%. At 18 months DLCO was 66.6% ±16%. 3 months post discontinuation of therapy DLCO was 65.5% ± 22.8%. There was no significant decline in DLCO (P<0.05) at 12 months, 18 months and 3-month post drug discontinuation (Figure 3).
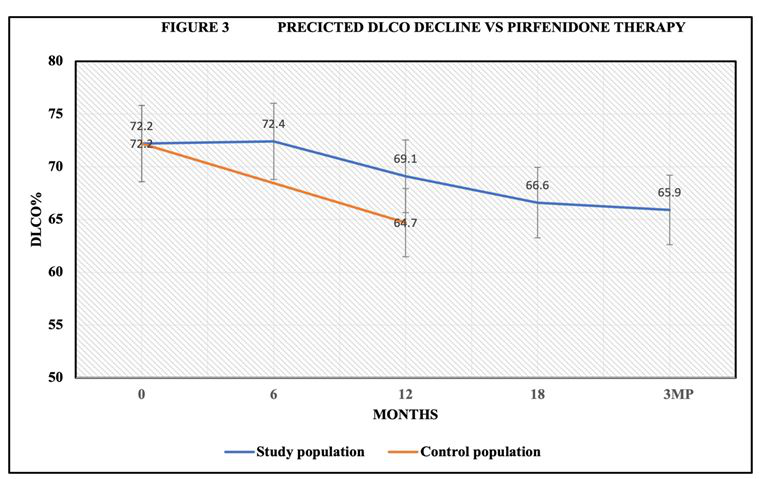
Figure 3:Pirfenidone reduced rate of DLCO decline during 18 months of therapy compared to control population. Control CWP/PMF received standard care in pulmonary practice.
Pulmonary function studies confirmed a beneficial effect on FVC, FEV1 and DLCO during pirfenidone administration. The rate of decline in lung function was significantly decreased during pirfenidone therapy when compared to control population of miners.
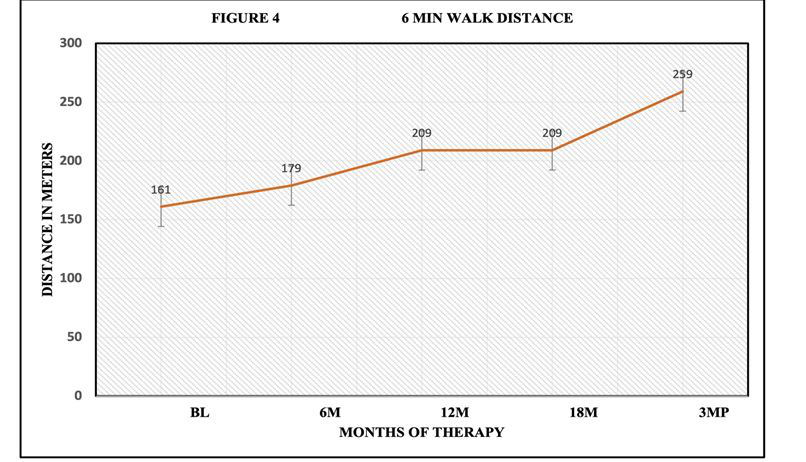
Figure 4:6-minute walk distance improved during Pirfenidone administration. Baseline distance was 161 m at screening and improved to 209 m following 18 months of therapy and continued to show improvement post discontinuation at 3MP.
6 Minute Walk Test was performed every 6 months to include oxygen saturation study as well as documentation of distance walked in meters within 6 minutes. 6-minute walk test: average at base line 161 m, 6-month 179 m but 2 required oxygen @ 2l/min, 1@ 3l/min. 12-month average was 209 m with 2 subjects on oxygen at 2l/min and ,1 @ 3L/min. Patients completing 18 months averaged 209 m with 2 subjects on oxygen. 3-month post drug discontinuation, subjects averaged 259 m, but 3 subjects were unable to walk due to arthritis/weakness. 3 subjects required O2 @ 2L/min, 1 subject @ 3L/min. There was no relationship of patient oxygen requirements and prior covid infection recorded during the study. A positive impact of 6-minute walking distance was observed. (Figure 4). SGRQ scores were unchanged compared to baseline during trial but increased following discontinuation of antifibrotic therapy (Figure 5).
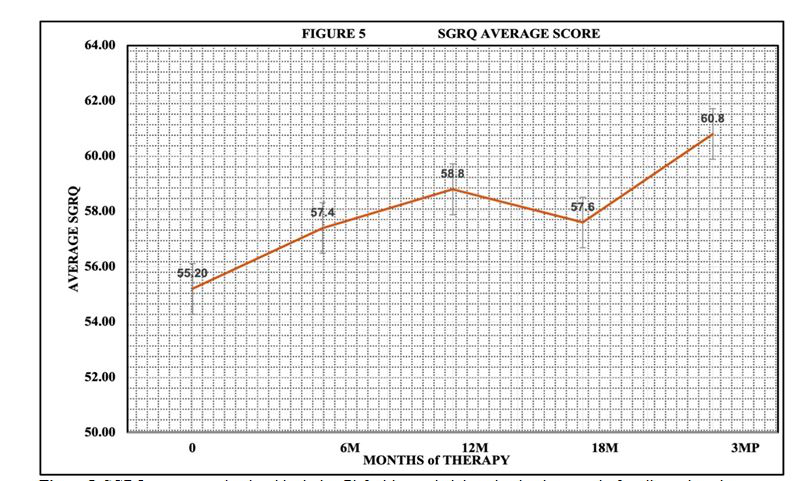
Figure 5:SGRQ scores remained stable during Pirfenidone administration but increased after discontinuation (3MP).
Additional data analysis included the perceived dyspnea and exertion scale at rest and exercise. Baseline status recorded a dyspnea index of 1.4 rest and 3.4 with exercise. After 6 months of therapy, index was 2.0 at rest and 3.1 with exercise. Following 12 months of therapy, index was 1.68 at rest and 3.3 with exercise. Selected patients completing 18 months of therapy recorded a rest index of 1.7 and 3.2 with exercise. After completion of study, 3 months post last dose, dyspnea index at rest was 1.1 and 3.7 with exercise. Baseline exertion rating recorded 0.7 at rest and 2.8 with exercise. 6-month patients recorded 1 at rest and 2.7 with exercise. Following 12 months of therapy, exertional dyspnea was quantitated at 0.6 at rest and 2.7 with exercise. Patients completing 18 months of therapy recorded an average of 0.9 at rest and 2.6 with exercise. Following completion of the therapy 3 months post last dose, exertional score was 1.1 at rest and 3.6 with exercise. The dyspnea and exertion index with exercise were stable during trial, but exertion dyspnea increased after pirfenidone was discontinued, consistent with a beneficial impact of pirfenidone administration (Figure 6). Serial CT scans of thorax failed to demonstrate significant progression of CWP/PMF during the trial. CT scans were compared to initial CT scans and reviewed by radiologist and principal investigator.

Figure 6:Dyspnea index at rest remained stable during and after discontinuation of therapy. Dyspnea index with exertion however increased after therapy discontinuation and was consistent with SGRQ scores.
¬Adverse events reported during the study were consistent with package insert. 55 subjects signed informed consent with 50 enrolled: 9 experienced significant photosensitivity; 3 responded to dosage adjustment and 6 discontinued due to grade 3 (severe) photosensitivity. 12 reported significant dyspepsia with 4 responding to dosage adjustments with 8 discontinued. Exacerbation of respiratory symptoms occurred in 9 patients with 5 covid positive. 1 experienced encephalopathy/confusion and discontinued (not related to therapy). 1 withdrew following diagnosis of liver cancer requiring treatment. 1 withdrew due to increasing shortness of breath. 7 withdrew consent. 1 expired due to pulmonary emboli/Covid infection not related to therapy. A total of 10 Covid positive were documented during the study with 4 hospitalizations. 1 withdrew following lung transplant. A total of 19 patients were withdrawn due to described adverse events.
Discussion
Pirfenidone is an orally active, small molecule that exerts broad antifibrotic activity across organ systems has been demonstrated in more than 40 animal studies in models of fibrosis, including fibrosis of the liver, heart, and kidney as well as several models of lung fibrosis. Nobel et al. [10] demonstrated pirfenidone efficacy in reducing rate of lung function decline in IPF. DuBois et al. [4] felt pathogenesis of IPF remains incompletely understood, the current view is that a series of microinjuries to the alveolar epithelium results in release of profibrotic mediators, causing fibroblast and myofibroblast proliferation, organization into fibroblastic foci, and excessive collagen deposition and accumulation. Flaherty et al. [5] reviewed the rate of decline of FVC in patients with interstitial lung disease treated with anti-fibrotic therapy. An average FVC decline of 187 ml per year documented in placebo control patients. Antifibrotic therapy reduced rate of decline by 57%. Nonclinical studies conducted in vitro as well as data derived from animal models of fibrosis provide evidence that pirfenidone reduces levels of profibrotic growth factors and cytokines, lessens collagen deposition, and reduces interstitial fibrosis-all physiological elements thought to be dysregulated in pulmonary fibrosis. It was reported by Yao et al. [13] that exposure to coal dust is associated with accumulating macrophages and apoptosis of alveolar macrophages in CWP. TNFβ expression was increased in this population which increased autophagy and apoptotic index in CWP. Perret et al (11) reported human models are required to explore potential re-purposing of antifibrotic therapy with potential efficacy in this group.
Coal mine dust causes a spectrum of lung disease collectively termed coal mine dust lung disease (CMDLD). These include interstitial lung disease, silicosis, mix dust pneumoconiosis, dust related diffuse fibrosis, and chronic obstructive pulmonary disease [6]. Treatment of CWP/PMF has been symptomatic and those with end-stage disease are candidates for lung transplantation. Because CWP/PMF cannot be cured, reduction of dust exposure was the only alternative. Spirometry testing [2] in coal miners with PMF and documented airflow obstruction in 32.3% of these individuals. The rate of lung function decline in aging adults was examined in the British Medical Journal volume and recorded an average decline of FEV1 of 43.5 mL with age-related reduction of FVC up to 65 mL/yr. Cohen et al. [3] reviewed respiratory disease in miners with progressive massive fibrosis. There has been significant increase in proportion of individuals with progressive massive fibrosis. Researchers noted an increased mortality and loss of lung function in PMF. The average annual rate of decline in FEV1 ranged between 92 mL and 109 mL per year and had 3-fold greater risk of dying from progressive airflow obstruction [8]. Mazurek et al. [9] reviewed years of potential life lost expectancy and documented decedents age > 25 with CWP/PMF lost more years of life relative to their life expectancies.
Blackley et al. [2] documented an increase in lung transplantation for CWP. Mean age was 56 with an FEV1 35% and FVC 53% of predicted. Almberg et al (1) examined the incidence of PMF was greatest in the central Appalachian area with 84% of cases in West Virginia, Kentucky, Pennsylvania, and Virginia. Cohen et al. [3] reviewed lung pathology in U.S. coal workers with PMF. Silicosis lesions were present with interstitial pulmonary fibrosis found in majority of cases. Areas of usual interstitial pneumonia was present without evidence of neoplasm or active infection from bacteria or fungi. Coal dust has been shown to stimulate the release of cytokines that are important in lung inflammation and fibrosis, including tumor necrosis factor alpha and IL-6 [7]. Areas of fibrotic reaction occur with local occlusion of airways and pulmonary vessels. There is however an individual response to dust reticulation which is representative of inflammatory reactions often associated with oxidative damage in the lung tissue [14].
Conclusion
This study suggests individuals with coal workers pneumoconiosis and progressive massive fibrosis (CWP/PMF) demonstrate progressive reduction of airflow (FEV1) rather than restrictive lung disease. Older literature suggested a predominate restrictive disorder. A corresponding reduction of DLCO consistent with fibrosis and parenchymal scarring is present. It was felt pirfenidone decreased the inflammatory process associated with pulmonary fibrosis in CWP/PMF. Serial CT thorax during study failed to demonstrate significant radiographic progression. Pirfenidone therapy reduced the rate of decline of FVC, FEV1, and DLCO in this population. SGRQ scores were stable during antifibrotic therapy (P<0.05) but did increase following discontinuation. 6-minute walk test improved during trial and corresponded to dyspnea and exertion index. Dyspnea and exertion index increased following drug discontinuation confirming a positive outcome and beneficial effect of pirfenidone administration in CWP/PMF. Pirfenidone was effective in reducing loss of lung function in fibrotic occupational lung disease and should be considered an option to reduce probability of lung transplant.
Abbreviations
PFD: pirfenidone; CWP/PMF: Coal workers pneumoconiosis with progressive massive fibrosis; CMDLD: Coal mine dust lung disease; IPF: idiopathic pulmonary fibrosis; PFT: pulmonary function test; FEV1: Forced expiratory volume in 1 second; FVC: Forced vital capacity; DLCO: Diffusion capacity of lung for carbon monoxide; SGRQ: Saint George’s Respiratory Questionnaire; 6MWT: 6-minute walk test; CT of thorax: Computerized tomography of chest; IL-6: Interleukin 6; TNF-β: Tumor necrosing factor Beta
Acknowledgements
TRIAL REGISTERY: ClinicalTrials.gov; No: NCT04461587.
Investigator Initiated Study ML42227; Funding provided by Genentech, a Member of Roche Group. Advarra IRB approved as phase 2 study on 6/22/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. Almberg KS, Halldin, CN, Blackley DJ, Laney AS, Storey E, Rose CS, et al. Progressive massive fibrosis resurgence identified in US coal miners following filing for Black Lung benefits, 1970-2016. Ann Am Thoracic Soc. 2018;15(12):1420-1426.
2. Blackley DJ, Halldin CN, Cummings KJ, Laney AS. Lung Transplantation is Increasingly Common Among Patients with Coal Workers’ Pneumoconiosis. Am J Ind Med. 2016; 59(3): 175–177.
3. Cohen RA, Petsonk EL, Rose C, Young B, Regier M, Najmuddin A, et al. Coal Workers with Rapidly Progressive Pneumoconiosis Implicates Silica and Silicates. Am J Respir Crit Care Med. 2016; 193(6):673-680.
4. DuBois, Roland; An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. European Respiratory Review. 2012; 21: 141-146.
5. Flaherty K, Wells AU, Cottin V, et al. Nintedanib in progressive interstitial lung disease: data from the whole INBUILD trial. European Respir J. 2022; 59 (3) 20054538.
6. Kurth L. Prevalence of Spirometry-defined airflow obstruction in never smoking working US coal miners by pneumoconiosis status. Occup Envioron Med. 2020.77(4) :265-267.
7. Lee JS, Shin JH, Lee KM, Hwang JH, Baek J, Kim JH et al. Serum levels TGF-B1 and MCP-1 as biomarkers for progressive coal workers’ pneumoconiosis in retired coal workers: a three -year follow up study. Industrial Health. 2014; 52: 129-136.
8. Liso SY, Lin X, Christiani DC. Occupational exposure and longitudinal lung function decline Am J Ind Med. 2015; 58: 14-20
9. Mazurek J.M.; Wood,J.; Blackley, D.J.; Weissman, D.N.; Coal Workers’ Pneumoconiosis-Attributable years of Potential Life Lost Before Age 65-United States, 1999-2016. MMWR Morbidity Mortality Wkly Rep.2018 3; 67(30):819-824.
10. Noble PW, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomized trials. Lancet. 2011; 377: 1760-1769
11. Perret JL, Plush B, Lachapelle B, Hinks TS, Walter C, Irving L, et al. Coal mine dust in the modern era. Respirol. 2017;22(4): 662-670.
12. Scott AS, Weissman DN. Respiratory Diseases Caused by Coal Mine Dust. J of Occ Env Med. 2014;(56): S18-S22.
13. Yao SQ, Yang NW, Guo FF, Qin B, Zhu XP, Dong ZG, et al. Expression of type 1 and type 2 cytokines from serum of coal miners and the evaluation of surveillance of coal workers’ pneumoconiosis at earlier stage.
14. Yucesoy B, V Johnson, Kissling GE, Kashon ML, Slaven J, Germolec D, et al. Genetic susceptibility to progressive massive fibrosis in coal miners. European Respir J. 2008; (6): 1177-83.
Received:February 13, 2023;
Accepted: March 06, 2023;;
Published: March 07, 2023.
To cite this article : Emory H Robinette, Retha Robinette. Pirfenidone Therapy in Coal Workers’ Pneumoconiosis with Pulmonary Fibrosis and Associated Functional Impairment. European Journal of Respiratory Medicine. 2023; 5(1): 372 - 376. doi: 10.31488/ EJRM.138.
© The Author(s) 2023. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
