Research Article/ Open Access
DOI:10.31488/EJRM.123
Prospective, Non-Controlled Pilot Study to Evaluate the Efficacy and Safety of Cefditoren Pivoxil in COVID-19 Patients with Mild to Moderate Pneumonia
Cristóbal M. Rodríguez Leal1, Mercedes Gimeno del Sol*2, Mª Pilar Coronel Granado3, Mª Ángeles Campos Fernández de Sevilla4, Martín S. Ruiz Grinspán1, on behalf of CATCH working group
1.Emergency Department, Henares University Hospital, Coslada, Madrid, Spain
2.Clinical Development. Department, Meiji Pharma Spain, Alcalá de Henares, Madrid, Spain
3.Scientific Department, Meiji Pharma Spain, Alcalá de Henares, Madrid, Spain
4.Pharmacy Department, Henares University Hospital, Coslada, Madrid, Spain
*Corresponding author: Mercedes Gimeno del Sol, Clinical Development Department, Meiji Pharma Spain S.A. Avda de Madrid 94, 28802 Alcalá de Henares, Madrid, Spain.
Abstract
One of the strategies to fight against COVID-19 is the “repositioning” of drugs that are already approved to treat other diseases. There is published evidence that cefditoren, a third-generation oral cephalosporin for respiratory diseases, has anti-inflammatory effects as well as high capacity to bind to SARS-CoV-2 protease. In this context, it was considered of interest to test the efficacy of cefditoren through a pilot study in patients with confirmed COVID-19 seen at the Emergency Unit of a public hospital, and susceptible to ambulatory follow-up. The primary outcome was the clinical evolution at days 2, 7, 14, and 28 after discharge based on a modified WHO score and a standardised questionnaire. All 20 patients included had a fever and radiological symptoms of mild-moderate pneumonia; other symptoms were cough (80%), fatigue (80%), myalgia (75%), headache (65%) and arthralgia (60%), with the mean number of symptoms being 6.3. Among the patients that completed the 7 days of treatment, there was a clear improvement in the scores, with statistical significance (p<0.05) from day 2 onwards. At the end of follow-up, 79% of patients were classified as “ambulatory with no limitation of activities”. One patient with poor evolution was considered a clinical failure. Ambulatory follow-up would contribute to alleviating pressure on hospitals and reducing costs to the healthcare system. These preliminary results should be studied more in depth in an adequate population.
Key Words: COVID-19, cefditoren, pneumonia, SARS-CoV-2, ambulatory care, pilot study
Introduction
The global pandemic of novel coronavirus disease 2019 (COVID-19) began in Wuhan, China, in December 2019, and has since spread worldwide. The novel coronavirus is now referred to as severe and critical acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) [1].
SARS-CoV-2 is transmitted through the respiratory tract and could induce pneumonia [2,3]. Bacterial co-infection in the setting of viral pneumonia is known as major cause of mortality [4].
Nowadays, there is only conditional marketing authorisation in the European Union for Remdesivir, an antiviral with limited effect in the natural course of pneumonia [5]. Taking into account that global vaccination is a medium-term option among the possible strategies, the so-called “repositioning”, that is, the use against SARS-CoV-2 of drugs that are already approved to treat other diseases, is established. In consequence, there is a need to evaluate existing drugs with potential efficacy for COVID-19 through clinical trials [6].
Cefditoren (CDN) is a third-generation cephalosporin for oral administration in the form of cefditoren pivoxil (CDN-PI), a prodrug which is hydrolysed by intestinal esterases releasing the active form into the bloodstream. Cefditoren was discovered and patented by Meiji Seika Pharma, Co, Ltd. (Japan).
Cefditoren has a broad spectrum of antibacterial activity and is particularly active against S. pneumoniae, H. influenzae, M. catarrhalis, methicillin-susceptible Staphylococcus aureus, and S. pyogenes, the main pathogens involved in respiratory tract and skin and soft tissue infections. Particularly relevant is the activity of CDN against S. pneumoniae strains with decreased susceptibility to penicillin. In this field, CDN activity is superior to that of the remaining oral cephalosporins and equivalent to that of cefotaxime and ceftriaxone [7].
Cefditoren pivoxil was authorised in Japan in 1994. In Europe, the product was approved in Spain in 2004 as a first market and has subsequently been registered in several European countries. Currently the product is approved in near 40 countries worldwide.
The results of clinical trials with CDN on community-acquired pneumonia showed percentages of efficacy 85% and the microbiological efficacy demonstrated in clinical trials, with a bacterial eradication rate over 80%, consolidates the data on its excellent antimicrobial activity against the bacteria more closely related to respiratory infections including resistant strains [7,8].
In patients with exacerbations of COPD it seems that high levels of IL-6, a mediator of lung inflammation, could predict a worse prognosis in these patients. The use of CDN is associated with a marked decrease in circulating levels of IL-6 and other pro-inflammatory cytokines and mediators of epithelial damage, such as Krebs von den Lungen-6 (KL-6) [9]. This is possibly a surrogate indicator of its potent antibacterial activity that determines a rapid decrease in the inoculum and thus less inflammatory activity [10].
In COVID-19 patients, the elevated inflammatory cytokines and other inflammatory mediators present suggest that a cytokine storm, also known as cytokine release syndrome (CRS), may play a major role in the pathology of this disease [11]. The elevated cytokine levels may also be responsible for the lethal complications of COVID-19. Specifically, the potential of IL-6 pathway inhibition in COVID-19 pneumonia is supported by studies in which elevated concentrations of IL-6 have been reported, together with several laboratory abnormalities suggestive of hyperinflammation, especially in patients admitted to intensive care units [12,13]. Therefore, the interleukin-6 (IL-6) blockade has been proposed as one of the strategies to manage COVID-19-induced CRS [14].
KL-6 is mainly produced by damaged or regenerating alveolar type II pneumocytes. Serum concentrations of KL-6 were only elevated in patients with severe pulmonary involvement, revealing a prognostic value and supporting the potential usefulness of KL-6 measurement to evaluate COVID-19 patients’ prognosis [15].
A population of laboratory-confirmed COVID-19 patients was tested for 39 respiratory pathogens. In total 94% patients were co-infected with one or more pathogens. Bacterial co-infections were dominant in 92% of COVID-19 patients with S. pneumoniae being the most common, followed by K. pneumoniae and H. influenzae. Most co-infections occurred within 1–4 days of onset of COVID-19 disease. In a retrospective study, secondary infection was observed in 50% of non-survivor patients [16].
On the other hand, a bioinformatic approach used the structure for COVID-19 protease as a receptor and a selection of 9 antivirals and 21 antibiotics used for respiratory infections as ligands, based on their chemical structures. CDN was one of the antibiotics tested and the findings suggest that cefditoren has the highest binding capacity and inhibitory potency among the antibiotics tested and superior to several antivirals [17].
Considering the above information and the current state of knowledge against SARS-CoV-2, we have considered it to be of interest to test the efficacy of CDN through a pilot study in a number of patients with COVID-19 that will be subject to ambulatory follow-up.
Materials and Methods
Ethics and registration
The trial was conducted in accordance with Good Clinical Practice and in compliance with the principles of the Declaration of Helsinki. The protocol was approved by the Ethics Committee for Research with drugs from Hospital Universitario La Paz, Madrid, Spain (approved on 19 of November 2020, minutes no. 22/2020). All patients provided their written informed consent to participate in this trial. The study was registered at ClinicalTrials.gov with the identifier NCT04709172.
Design
This was a prospective, pilot, low-intervention, case series, single center, non-controlled study. Patients arrived at the Emergency Unit of the hospital and, after verifying the selection criteria and obtaining informed consent, started treatment with the test product, CDN-PI 400 mg tablets every 12 hours for 7 days. If necessary, the patient could remain under observation for 48-72h and based on the evolution and according to the criteria established in Table 1, it was decided if the patient could be discharged or if he/she needed to be hospitalised. If hospitalisation was needed, the patient was withdrawn from the study, and an alternative treatment was prescribed.
Table 1:Criteria for discharge/admission
| DISCHARGE All the following must be met |
OBSERVATION All the following must be met |
ADMISSION Must meet some |
|---|---|---|
| Respiratory rate ≤ 22 bpm | Clinical situation expected to improve in 48-72
hours: • In the absence of pulmonary consolidation or with unilobar consolidation; but with intense breakdown, dyspneic sensation, SaO2 < 95% upon arrival with the patient’s usual FiO2 (but greater than 90%) or FR greater than 22 bpm upon arrival. OR • In the presence of multilobar lung consolidation. |
Tachypnoea at rest (more than 30 bpm). |
| SaO2 ≥95% Absence of dyspneic sensation at rest and with effort. No desaturation or tachypnoea after walking 50 steps |
SaO2 < 90% or pO2 < 60 with patient’s usual FiO2 |
|
| Δ SOFA < 2 | Δ SOFA < 2 | Δ SOFA < 2 |
| CURB-65 = 0-1 | CURB-65 = 0-1 | CURB-65 ≥ 2 |
| Lung consolidation absent or not multilobar | Not meeting any admission criteria | |
| PCR positivity: number of cycles > 25 (illustrative criterion) | Hyperinflammatory response that indicates the need for biological treatment: IL-6 > 40, ferritin > 1000, etc. | |
| Acute venous thromboembolic disease | ||
| Criteria for severe pneumonia (IDSA 2007). |
CURB-65 [18]: score for pneumonia severity (Confusion, Blood urea nitrogen [BUN], Respiratory rate, Blood pressure and Age 65 years); FiO2: fraction of inspired oxygen; PCR: Polymerase chain reaction; SaO2: oxygen saturation; SOFA [19]: Sequential Organ Failure Assessment. Patients over 50 years of age are at greater risk, but it is not a determining factor itself.
After discharge, patient follow-up lasted for 4 weeks by telephone, always using the same questionnaire for all patients to know their condition and to evaluate the need for a new visit to the Emergency Unit. The study schedule is shown in Figure 1.
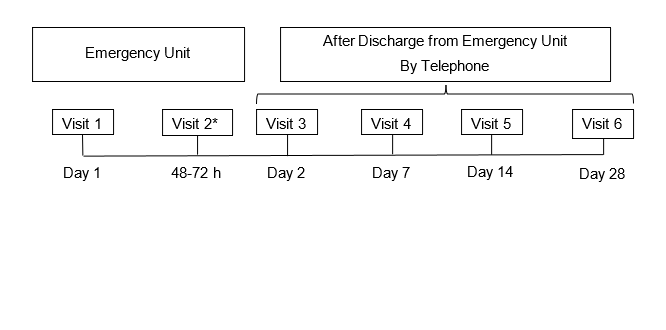
Figure1:Study schedule
*To be performed only in case the patient remains under observation at the Emergency Unit.
During the follow-up calls, patient improvement was assessed using a standard clinical evaluation questionnaire [20], and based on it, a score was assigned using the scale developed by the World Health Organization (WHO) [21]. This questionnaire allowed the clinician to evaluate via telephone contact the condition of the patient after discharge and the need to return to the Emergency Unit for additional medical examination. In summary, the questionnaire consists of 16 questions divided into subjective (n=11) and objective (n=5) ones. Subjective questions are in turn divided into major and minor ones. The more questions answered yes, the worse the patient’s condition. The patient would be recommended to return to the Emergency Unit if he/she answered yes to two or more questions (except if all of them are minor ones) or to one major question. In turn, the WHO score was adapted to the characteristics of the study (referred to in this study as modified WHO, mWHO) and the condition of “uninfected” was not considered as it was not possible to be assessed through telephone follow-up. No additional PCR test was performed after discharge to confirm a negative result.
Concomitant medication for COVID-19 according to hospital protocol was permitted provided that it does not interfere with the evaluation of the study drug.
The previous medication administered in the seven days prior to inclusion and all concomitant medication administered during the study was recorded.
Study population
Eligible patients were men and women aged ≥ 18 years with a positive real time polymerase chain reaction (RT-PCR) or rapid antigen test for SARS-CoV-2 (Panbio™ Abbott COVID-19 Ag Rapid Test) at inclusion or within the previous 48 hours and with the following condition: clinical and radiological symptoms of mild-moderate pneumonia; fever 37.7ºC at admission or related; SatO2 > 94% and respiratory rate < 24 at admission; able to take oral medication; negative for HIV. Exclusion criteria included need of oxygen at the time of inclusion; alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 5 times the upper normal limit during screening; QTc interval prolongation > 450 ms; moderate or severe renal impairment (creatinine < 50 ml/min); severe hepatic impairment (Child-Pugh C); pregnancy or childbearing; known hypersensitivity to beta-lactams; malabsorption or swallowing problems; inability to understand and follow study procedures; or treatment with other drugs active against SARS-CoV-2 within 24 hours prior to initiating the study.
Outcome measures
The primary outcome measure was the clinical evolution at days 2, 7, 14 and 28 after discharge, using the mWHO Score and the Clinical evaluation questionnaire. As secondary outcomes, the need for hospitalisation due to treatment failure, an additional visit to the Emergency Unit due to worsening of COVID-19 and the occurrence of adverse events were evaluated.
Statistics
Descriptive statistics was done using percentages for qualitative variables and with means and standard deviations for quantitative variables. Due to the small sample size, medians and interquartile ranges (p25-p75) were also used. For the same reason, the inferential statistics of the quantitative variables have been supported by the Mann Whitney test for comparisons between independent groups or the Wilcoxon test for repeated samples (pre-post). In the case of categorical variables, the Chi2 test was used, which had to be completed or corrected by Fisher’s exact test in all cases due to the small sample size. A type I error of 5% was accepted. The analyses were performed with SPSS V14 (SPSS Inc, Chicago IL, 2007).
Results
The study was designed as a pilot clinical trial with a planned sample size of 30 patients and started in January 2021. Due to the huge increase of the vaccination rate among the population, the number of cases of infection, and in consequence the number of patients included in the study dropped dramatically, to the extent that in mid-August it was decided to discontinue recruitment. In the time period between January 2021 (during the so-called third wave, with the alpha variant as dominant) and August 2021 (during the fifth wave, with the delta variant as dominant) a total of 20 patients were recruited.
All patients that received at least one dose of CDN form the “intention to treat” population (ITT). Of the 20 recruited patients, 14 completed 7 days of treatment with CDN (14 doses) according to protocol and constitute the analysis population “per protocol” (PP) that was the main population for efficacy assessment. Population for safety included all patients who have received at least one dose of CDN. The distribution of the patients is shown in Figure 2.
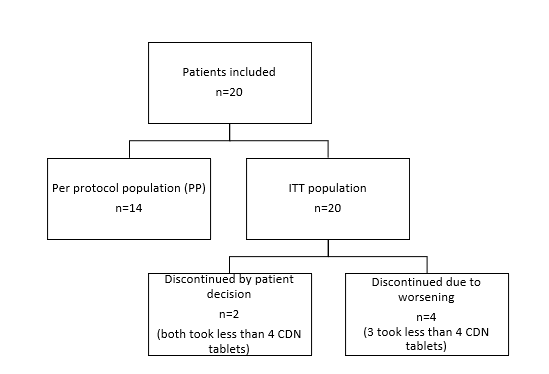
Figure2:Distribution of patients
The patients had a mean age of 47 years and there were no patients over 67 years old. The female/male percentage was 55%/45% and 85% of the patients were Caucasian. With respect to occupations, 90% had active paid employment. The sample had a mean weight of 82.56 kg and a height of 1.66 m. The mean BMI was 29.72 kg/m2 and 35% of the patients were obese (BMI>30 kg/m2). As for the presence of risk factors that may influence a poor evolution of COVID 19, the most frequent ones were obesity (n=7), hypertension (n=7), ex-smoker or active smoker (n=5) and diabetes (n=3).
The laboratory diagnosis of SARS COV 2 was done by RT-PCR in 4 patients and by rapid antigen testing in 17 patients. In one patient both techniques were performed, and both were positive. The main symptom at baseline was fever (it was an inclusion criterion). Only one patient had a fever as the only symptom, while the other 19 had several, with the mean at baseline (ITT) being 6.3 symptoms/patient. Besides fever, the most frequent symptoms were cough (80%), fatigue (80%), myalgia (75%), headache (65%) and arthralgia (60%). There were no cases of rib pulling, confusion, gait difficulties, seizures or lymphadenopathies. A summary of the signs and symptoms at baseline is shown in table 2.
Table 2:Signs and symptoms at baseline (n=20)
| All patients, mean (SD) | |
|---|---|
| Temperature | 38.2 (0.49) |
| SBP/DBP | 130.10/80.80 (19.14/10.59) |
| Heart rate | 98.50 (15.74) |
| Respiratory rate | 19.00 (4.67) |
| SatO2 | 96.40 (1.63) |
| Symptoms affecting ≥ 25% of patients, n (%) | |
| Cough | 16 (80%) |
| Fatigue | 16 (80.0%) |
| Myalgia | 15 (75.0%) |
| Headache | 13 (65.0%) |
| Arthralgia | 12 (60.0%) |
| Thoracic pain | 9 (45.0%) |
| Diarrhoea | 7 (35.0%) |
| Sore throat | 7 (35.0%) |
| Anosmia | 6 (30.0%) |
| Ageusia | 6 (30.0%) |
| Anorexia | 6 (30.0%) |
SBP/DBP: systolic blood pressure/diastolic blood pressure
Auscultation was normal in 45% of ITT and 50% of PP patients. The predominant sign was crackles (around 40% of patients) (Table 3).
Table 3:Findings on auscultation and X-ray (overall population, n=20)
| Finding | Right lung | Left lung | |
|---|---|---|---|
| Auscultation | |||
| Rhonchus | 2 (10.0%) | 1 (10.0%) | |
| Wheezing | 1 (10.0%) | 1 (10.0%) | |
| Crackles | 8 (40.0%) | 7 (35.0%) | |
| Others | 1 (10.0%) | 1 (10.0%) | |
| Normal | 11 (55.0%) | 12 (60.0%) | |
| X-ray | |||
| Upper lobe | 3 (15.0%) | 3 (15.0%) | |
| Mid lobe | 4 (20.0%) | - | |
| Lower lobe | 15 (75.0%) | 15 (75.0%) |
At baseline, most patients had very low score rates of 0-1 in all CURB [18], SOFA [19] and HEWS [22] severity scales considered. As for the mWHO score, all patients were rated with a score of 2 (ambulatory with limitations).
As for the X-ray image, the lower lobes were the most affected, with 75% and 85% of patients in ITT or PP, respectively. Furthermore, all patients had radiographic signs of pneumonia. The dominant radiological pattern was of interstitial infiltrates and ground glass opacity, being present in 50% of ITT patients and 43% of the PP population. This is the most frequent radiological pattern in pneumonia caused by COVID-19. The only patient who had lung ultrasound available exhibited the presence of B lines in the lung fissures, which is an early sign of pneumonic involvement, although it is not specific for COVID-19 pneumonia. (Table 4).
Table 4:Pulmonary findings on image exam (ITT, n=20)
| N | (%) | |
|---|---|---|
| Parenchymal involvement | 2 | 10.0 |
| Consolidation | 1 | 5.0 |
| Infiltrated, without specifying | 3 | 15.0 |
| Interstitial | 8 | 40.0 |
| Opacity | 1 | 5.0 |
| Pseudonodular | 1 | 5.0 |
| Vascular weft | 1 | 5.0 |
| Ground glass opacity | 2 | 10.0 |
| B lines (ULTRA) | 1 | 5.0 |
| Total | 20 | 100.0 |
11 had improved on their score (78.4%), 3 remained in the same condition (21.4%), and none had worsened.
None of the patients presented anaemia or had haematocrit or haemoglobin below normal levels. Five patients had clear lymphopenia (<900 cells/mm3). Blood glucose values were not very high (percentile 75% was 128 mg/dl). It was quite surprising that ferritin values were high (percentile 75% was 818 ng/ml, although only available in 7 patients) and those of C reactive protein were not very high (percentile 75% was 50 mg/l). Only 3 patients had D-dimer available. Variables with relevance in the prognosis of COVID-19, such as creatinine, were not elevated in any patient. LDH was available only in 7 patients and 2 of them had high values, while ALT was high in 3 of 20 patients and AST was elevated in 5 of 19 patients.
Blood cultures were made from the 20 patients, with 19 being negative and 1 contaminated; 20 pneumococcal urine antigen tests were performed, 19 of which were negative and 1 could not be processed. Respiratory samples also had negative results.
At baseline, most patients had very low score rates of 0-1 in all CURB [18], SOFA [19] and HEWS [22] severity scales considered. As for the mWHO score, all patients were rated with a score of 2 (ambulatory with limitations).
The 14 patients included in the PP population that completed the study according to protocol showed a clear improvement in the WHO score with median values that reached statistical significance from day 2 onwards (Table 5). At the end of the study, 11 had improved on their score (78.4%), 3 remained in the same condition (21.4%), and none had worsened.
Table 5:mWHO COVID-19 Score to evaluate clinical improvement in PP population (n=14). Only the upper part of the scale is shown
| Phone contact (days after discharge (± 1d) | |||||
|---|---|---|---|---|---|
| baseline | Day 2 | Day 7 | Day 14 | Day 28 | |
| Ambulatory with no limitation of activities | - | 8 (57.1%) | 8 (57.1%) | 9 (57.1%) | 11 (64.3%) |
| Ambulatory with limitation of activities | 14 (100%) | 6 (42.9%) | 6 (42.9%) | 5 (35.7%) | 3 (21.4%) |
| mWHO score (median) (p value vs baseline) | 2 | 1 (0.005) |
1 (0.005) |
1 (0.004) |
1 (0.002) |
The clinical evolution questionnaire at follow-up is displayed in Table 6. On Day 2, a total of 31 questions were answered “yes”, compared to 1 on Day 28, with the differences being statistically significant.
Table 6:Clinical evolution questionnaire at follow-up (minor items in grey)
| Day 2 N=17 |
Day 7 N=14 |
Day 14 N=14 |
Day 28 N=14 |
|
|---|---|---|---|---|
| Overall number of questions answered “yes” | 31 | 13 | 5 | 1 |
| 1. Are you confused, drowsy, or dizzy (feeling light-headed)? | 1 (5.9 %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 2. Do you have chest fatigue doing light activities? | 2(11.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 3. Do you think you are breathing fast or accelerated? | 1 (5.9 %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 4. Do you have a cough? | 3 (17.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 5. When you cough, do you cough up mucus or phlegm that is green, brown, or pink? |
1 (5.9 %) | 2 (14.3%) | 0 (0.0%) | 0 (0.0%) |
| 6. Do you have a feeling of fatigue or muscle pain or a feeling of lack of strength in general? |
9 (52.9%) | 4 (28.6%) | 2 (14.3%) | 1 (7.1%) |
| 7. Do you have a sore throat? | 1 (5.9 %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 8. Do you have nausea? | 2 (11.8%) | 1 (7.1%) | 0 (0.0%) | 0 (0.0%) |
| 9. Have you had diarrheic stools? | 5 (29.4%) | 4 (28.6%) | 1 (7.1%) | 0 (0.0%) |
| 10. Have you had a feeling of shivering or fever? | 1 (5.9 %) | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) |
| 11. Overall, would you say that you are worse than when you were discharged from the Emergency Unit? |
1 (5.9 %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 12. Have you had a fever since discharge? | 1 (5.9 %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 13. Is fever controlled by taking the recommended antipyretics? | 1 (5.9 %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 14. Does the device show a systolic BP figure greater than 180 or less than 100 mmHg? |
0 (0.0%) | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) |
| 15. What is the number of pulsations that the device gives you? |
1 (5.9 %) | 1 (7.1%) | 0 (0.0%) | 0 (0.0%) |
Six patients did not complete the treatment: two of them by their own decision due to the appearance of adverse events (AEs) and 4 by decision of the specialist, due to the poor clinical evolution of COVID-19. Five out six took a number of CDN doses considered insufficient (≤4) and one of them completed 80% of the treatment and was considered a clinical failure.
Two out of the 6 patients that discontinued the study had received the first dose of the vaccine, one of them with Moderna one week before the study and the other one with Astra-Zeneca 10 weeks before; both patients took less than 20% of the treatment with CDN-PI.
As for the secondary outcomes among the completers’ population, there were 3 patients that made a new visit to the Emergency Unit, although only in one case was it due to worsening of COVID-19, and there were no hospitalisations due to clinical failure (Table 7).
A total of 35 AEs were recorded involving 13 patients; 22 were considered of mild intensity, 12 moderate and only one was severe. As for the causality, only 5 were considered possibly related: 1 case of epigastralgia, 1 case of hypertransaminasemia and 3 cases of diarrhoea.
Discussion
Spain is one of the EU countries most affected by the COVID-19 pandemic. From epidemiological studies performed, the overall mortality rate was 17.5% rising up to 36.5% in the subgroup of patients admitted to the ICU [23].
The majority of people infected with SARS-CoV-2 develop mild symptoms, not requiring hospitalisation or remain completely asymptomatic (approximately 80% to 90%). Depending on the time of the investigation, the cohort investigated and the virus variant [2, 23-26], the median incubation period is estimated to be between five and six days and 97.5% of symptomatic cases develop symptoms within 11.5 days of exposure [27]. Typically, people recover from COVID-19 after 2 to 6 weeks. While most people with COVID-19 recover and return to normal health, some people can have symptoms that last for weeks or even months after recovery from acute illness [28].
In light of the extent of the COVID-19 pandemic with its pressure on healthcare systems, especially in the face of evolving virus variants with potential increased transmissibility and altered disease characteristics, there is an urgent need for effective and safe therapies to save lives and to reduce the burden on healthcare systems. On the other hand, considering that the incidence of secondary infections by resistant pathogens is very high and worsens the prognosis, the objective of the specialist when faced with a COVID-19 patient is to avoid hospital admission [29] with the most effective treatment.
The population of our study was quite young, with a mean age of 47 years. All patients had active employment and did not have relevant comorbidities, with the exception of the obesity that was present in 35% of the sample and is considered a relevant factor for poor evolution [30,31]. All patients had a positive diagnosis for COVID-19 obtained through antigen rapid testing in 85% of the cases. As for signs and symptoms present at inclusion, all of them had a fever (inclusion criterion), around 80% had a cough and fatigue; other symptoms in 45-75% of the cases were myalgia, arthralgia, thoracic pain and headache; diarrhoea, sore throat, anosmia, ageusia and anorexia were also present in 30-35% of the participants. SOFA, CURB and HEWS scores were 0-1 at baseline.
Auscultation was normal in around 60% of the cases and the most frequent finding was crackles. On X-ray, all patients had radiographic signs of pneumonia with the lower lobes being the most affected. The dominant radiological pattern was of interstitial infiltrates and ground glass opacity, being present in 50% of the patients. Laboratory tests showed high values of ferritin and not very high values of protein C. Pneumococcal antigen was negative as well as respiratory samples and blood cultures analysed.
The definition of the clinical characteristics and comorbidity of patients with COVID-19 treated in Emergency Units helps to identify cases at risk of worsening, to predict outcomes, as well as to facilitate the implementation of preventive measures [31]. The characteristics of our patients were similar to those reported by other Spanish investigators elsewhere [30] in terms of signs and symptoms at baseline and concomitant conditions, although it has been reported that age and obesity showed a direct and independent association with a worse outcome; and in our series, age was not associated with a worse outcome, probably due to our patients being younger (47 vs 62 years old).
The evaluation of our patients’ improvement was based on the WHO score [21] that was adapted to the characteristics of the study (mWHO): the condition of “uninfected” was not considered as it was not possible to be assessed through telephone follow-up. A clinical questionnaire was also used for evaluation; this questionnaire was implemented with good results during the pandemic given the saturation experienced by Primary Care, to ensure a reassessment of those patients with mild symptoms that were discharged from the Emergency Unit. The patients were contacted by phone and participated in a brief interview to assess their evolution and, above all, establish the need for urgent, in-person reassessment. This kind of questionnaires [20] was proven very useful during a health emergency situation like the one we experienced in recent months.
In total, 6 patients did not complete the study, 2 of them due to adverse events considered not related to the study drug and 4 due to worsening of their condition. Of these 4, 3 patients took ≤ 20% of the treatment and could not be considered a clinical failure. The 14 patients that completed the study according to protocol showed a clear improvement in the mWHO score with median values that reached statistical significance from day 2 onwards. At the end of the scheduled follow-up, 11 had improved on the mWHO scale (78.4%), 3 remained in the same condition (21.4%), and none worsened.
At the time of emergency care, no patient had evidence of active bacterial infection and it is unknown how CDN could help in their favourable course. We could hypothesise that CDN could aid in avoiding later bacterial superinfection or acting as an antiviral [17]. New studies will be needed to discern this issue.
Vaccination has been shown to be highly effective at reducing severe illness and death from COVID-19. Safe and effective COVID-19 vaccines were developed in record time, but the virus is moving faster than the global distribution of vaccines. It is not known how effective vaccines will be against a new variant, as the world is asking itself nowadays about the new Omicron variant [32]. However, at the end of September 2021, almost 6-and-a-half billion doses had already been administered worldwide [33], and additional vaccine candidates are in development [34]. Nevertheless, the vast majority have been administered in high- and upper-middle-income countries [34], and that means that the majority of the world’s population still remains susceptible to SARS-CoV-2 infection and is at risk of developing COVID-19. Moreover, the duration and degree of protection against not only the disease but also against infection and transmission is still not well-defined. Under this situation, in the COVID-19 pandemic, drug repositioning has presented itself as an alternative to the time-consuming process of generating new drugs [35]. Anti-inflammatory agents have been associated with improved outcomes of hospitalised COVID-19 patients [36]. A continuing quest for specific inhibitors of pro-inflammatory cytokines brings promise for effective therapies designed for inflammatory and autoimmune disorders. As a part of this strategy, the immunomodulatory effects of some oral cephalosporins are being studied independently of their antimicrobial activity [37]. In a clinical study it was observed that the use of CDN in patients with exacerbations of COPD was associated with a marked decrease in circulating levels of IL-6 and other pro-inflammatory cytokines and mediators of epithelial damage [9]. Therefore, we consider that this anti-inflammatory action could contribute to the good evolution of the patients in our study and that this is an interesting mechanism of action that would be worth exploring more in depth.
In this pilot study, the majority of patients had a favourable outcome. There was an adequate evolution, with a good performance in the questionnaires at 28 days in almost all patients in the PP group. No deaths were recorded and most patients with a poor evolution did not receive enough doses of cefditoren. The treatment was well tolerated, with gastrointestinal side effects as the most frequent event, symptomatology that can be attributed to SARS-CoV-2 infection, because it is known that up to 20% of patients with COVID-19 can suffer this effect [31]. Despite the good results, the main limitation of our study is the lack of a control group and the small sample size, although it was designed as a pilot study, and we are aware that well controlled randomised clinical trials involving more patients are needed to confirm our promising results.
Conclusion
Cefditoren could be a valid option in the treatment of mild cases of patients affected by COVID-19 susceptible to ambulatory follow-up, thereby contributing to alleviating pressure on hospitals and reducing costs to the healthcare system. These preliminary results should be studied more in depth in an adequate population.
Catch* Working Group
Alonso Navarro R, Ampuero Martinich JD, Arroyo Linares V, Barrós González R, Belotto Fernández J, Coperías Zazo JL, El-Haddad Boufares N, Herrera Pacheco L, Galindo Martín MA, Gantes Nieto P, Gómez Sánchez L, González Camacho L, Mao Martín L, Martínez Avilés R, Mateas Moreno M, Rodrigo González S, Sanmartín Fenollera L.
CATCH*: COVID-19 Ambulatory Treatment with Cefditoren at Henares Hospital.
Acknowledgements
The authors would like to thank Dr Juan J. Granizo (Granadatos S.L.) for the statistical analysis of the results.
Funding
This work was supported by Meiji Pharma Spain S.A.
Conflicts of Interest
The authors declare not to have any conflict of interests.
References
1. Zhu X, Ge Y, Wu T, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005.
2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513.
3. Chu DK, Pan Y, Cheng SM, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020.
4. Guo G, Ye L, Pan K, et al. New insights of emerging SARS-COV-2: epidemiology, etiology, clinical features, clinical treatment, and prevention. Front Cell Dev Biol. 2020;8:410.
5. https://www.ema.europa.eu/en/medicines/human/EPAR/veklury, last access on 21/11/28
6. Jourdan JP, Bureau R, Rochais C, et al. Drug repositioning: a brief overview. J Pharm Pharmacol. 2020;72(9):1145-1151.
7. Giménez MJ, Aguilar L, Granizo JJ. Revisiting cefditoren for the treatment of community-acquired infections caused by human-adapted respiratory pathogens in adults. Multidiscip Respir Med. 2018;13:40.
8. Granizo JJ, Giménez MJ, Barberán J, et al. The efficacy of cefditoren pivoxil in the treatment of lower respiratory tract infections, with a focus on the per-pathogen bacteriologic response in infections caused by Streptococcus pneumoniae and Haemophilus influenzae: a pooled analysis of seven clinical trials. Clin Ther. 2006;28(12):2061-9.
9. Blasi F, Tarsia P, Mantero M, et al. Cefditoren versus levofloxacin in patients with exacerbations of chronic bronchitis: serum inflammatory biomarkers, clinical efficacy, and microbiological eradication. Ther Clin Risk Manag. 2013;9:55‐64.
10. .Sánchez Artola B, Barberán J. Cefditoreno: una realidad para el tratamiento de las infecciones comunitarias [Cefditoren: a reality for the treatment of community infections]. Rev Esp Quimioter. 2017;30(6):407‐412.
11. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250-256.
12. Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet.2020;395: 497–506.
13. Choy EH, De Benedetti F, Takeuchi T, et al. Translating IL-6 Biology Into Effective treatments. Nat Rev Rheumatol 2020;16(6):335-345.
14. Liu B, Li M, Zhou Z, et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452.
15. D’Alessandro M, Cameli P, Metella Refini R, et al. Serum KL-6 Concentrations as a Novel Biomarker of Severe COVID-19. J Med Virol. 2020;10.
16. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395(10229):1054-1062.
17. Dayer, Mohammad Reza. Old Drugs for Newly Emerging, COVID-19: Bioinformatic Prospective (2020).Available at https://www.researchgate.net/publication/339787179_Old_Drugs_for_Newly_Emerging_Viral_Disease_COVID-19_Bioinformatic_Prospective. Last access December 9, 2021.
18. Lim WS, Macfarlane JT, Boswell TC, et al. SCAPA: Study of Community Acquired Pneumonia Aetiology in adults admitted to hospital: implications for management guidelines. Thorax 2001;56:296–301.
19. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707-10. doi: 10.1007/BF01709751. PMID: 8844239.
20. Rodolfo Romero R, Alvarez V, Bibiano C et al. Guía de actuación de contingencia. Sección de Urgencias. Comités de emergencias. de hospitales.
21. [No authors listed] WHO R&D Blueprint novel Coronavirus.COVID-19 therapeutic Trial Synopsis. February 18, 2020, Geneva, Switzerland. Available at https://www.who.int › priority-diseases › key-action. Last access on November 30, 2021.
22. Gordo Vidal F, Abella Álvarez A. Early detection of the need for intensive care. Emergencias 2018; 30:350-353.
23. Cardinal-Fernández P, García E, Barberán J, et al. Clinical characteristics and outcomes of 1,331 patients with COVID 19: HM Spanish cohort. Rev. Esp Quim., 2021; 34(4): 342-351.
24. Pan X, Ojcius DM, Gao T, et al. Lessons learned from the 2019-nCoV epidemic on prevention of future infectious diseases. Microbes Infect. 2020;22(2):86-91.
25. Wu D, Wu T, Liu Q, et al. The SARS-CoV-2 outbreak: What we know. Int J Infect Dis. 2020;94:44-48. doi: 10.1016/j.ijid.2020.03.004.
26. Lauer SA, Grantz KH, Bi Q, et al The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172(9):577-582.
27. [No authors listed]. World Health Organization. Post COVID-19 condition (Long COVID). Available at: https://www.who.int/srilanka/news/detail/16-10-2021-post-covid-19-condition.
28. Suárez de la Rica A, Serrano P, de la Oliva R, et al. Secondary infections in mechanically ventilated patients with COVID 19: An overlooked matter? Rev Esp Quim. 2021; 34 (4) 330-336.
29. Udwadia ZF, Tripathi AR, Nanda VJ, et al. Prognostic Factors for Adverse Outcomes in COVID-19 Infection. J Assoc Physicians India. 2020;68(7):62-66. PMID: 32602683.
30. Gil-Rodrigo A, Miró Ò, Piñera P, et al; on behalf of the SIESTA research network. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias. 2020;32(4):233-241. English, Spanish. PMID: 32692000.
31. Hormanstorfer M, Borodowski H, Nelson K, et al. Prognostic value of static and dynamic biomarkers in COVID-19 patients: a prospective cohort study. Rev Esp Quim. 2021; 34 (4): 308-314.
32. [No authors listed]. https://www.paho.org/es/noticias/1-12-2021-oms-situacion-actual-relativa-variante-omicron. Last access December 12, 2021.
33. [No authors listed]. https://www.who.int/campaigns/vaccine-equity. Last access December 12, 2021.
34. [No authors listed]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Last access December 12, 2021.
35. Prieto Santamaría L, Díaz Uzquiano M, Ugarte Carro E, et al. Integrating heterogeneous data to facilitate COVID-19 drug repurposing. Drug Discov Today. 2021: S1359-6446(21)00438-4.
36. Kim MS, An MH, Kim WJ, et al Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020;17(12):e1003501.
37. Żyżyńska-Granica B, Trzaskowski B, Dutkiewicz M. et al. The anti-inflammatory potential of cefazolin as common gamma chain cytokine inhibitor. Sci Rep. 2020;10(1):2886.
Received: December 15, 2021;
Accepted: December 24, 2021;
Published: January 04, 2022.
To cite this article : Rodríguez Leal CM, Gimeno del Sol M, Coronel Granado MP, et al. Prospective, Non-Controlled Pilot Study to Evaluate the Efficacy and Safety of Cefditoren Pivoxil in COVID-19 Patients with Mild to Moderate Pneumonia. European Journal of Respiratory Medicine. 2022; 4(1): 249 - 257. doi: 10.31488/EJRM.123.
© 2022 Rodríguez Leal CM, et al.
