Research article/ Open Access
DOI:10.31488/EJRM.132
Safety and Efficacy of Nebulised Anti-Inflammatory Solution of Alkaline Hypertonic Ibuprofen (AHI) for Treatment of SARS-Cov-2 Infection: A Compassionate Study with a Comparator Arms
Galia Kalayan1, Ana Carolina Arias Cau2, Manuela del Valle Cabello2, Mariela Nuñez2, Romina Lumetto2, Nicolas Martinez Rios1, Luis Argarañas1, Néstor García3,4, Roxana Alasino4,5, Dante Beltramo4,5
1. Química Luar SRL – Córdoba, Argentina; 2- Hospital Orias, General San Martín, Jujuy, Argentina
2. Instituto de Investigaciones en Ciencias de la Salud-FCM (INICSA-CONICET), Córdoba, Argentina
3. Consejo Nacional de Investigaciones Científicas y Técnicas, CONICET, Godoy Cruz 2290, C1425FQB CABA, Argentina
4. Centro de Excelencia en Productos y Procesos (CEPROCOR), Córdoba, Argentina
*Corresponding author: Dra Roxana Alasino, Pabellón CEPROCOR, Complejo Hospitalario Santa María de Punilla, Córdoba, Argentina CP X5164. Dr. Dante Beltramo, Pabellón CEPROCOR, Complejo Hospitalario Santa María de Punilla, Córdoba, Argentina CP X5164.
Abstract
Background. This retrospective study evaluates the efficacy of inhalation therapy with alkaline hypertonic ibuprofen (AHI) in COVID-19 positive patients compared to a control group of patients who received conventional treatment. The study was carried out at the Orías Hospital in Jujuy Province, Argentina, from June to September 2020, with final follow-up on September 30. Methods. The study included 99 COVID-19 positive patients with moderate to severe disease (respiratory distress and/or hypoxemia). The control group of 62 patients were treated with the protocols in force at that time, oxygen, dexamethasone and enoxaparin. The group under evaluation comprised 37 patients treated with AH), in addition to standard treatment. Findings. Result shows that the treatment with AHI formulation is safe and effective. The mean respiratory rate (RR) of the patients went from 26.3 before treatment with AHI to 19.8 after treatment. On the other hand, the O2 saturation of patients before treatment with AHI showed a mean of 89.0%; at the end of treatment, the mean was 95.8%. In patients treated with AHI, on day 14, 85% had been discharged, while in patients not treated with AHI, only 36-37% were discharged on day 23. Patients with standard treatment, without AHI, show a mortality of 38.7%, distributed evenly in the 25 days of hospitalization. The mortality of patients treated with AHI was 8.1%, observed within the first 10 days of hospitalization. Interpretation. Results show that nebulization with AHI is an anti-inflammatory therapeutic alternative for the treatment of COVID-19 positive patients.
Key words: hypertonic alkaline ibuprofen, nebulization, COVID-19 treatment, anti-inflammatory
Introduction
The coronavirus disease 2019 (COVID 19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shows high rates of transmission and substantial mortality. The symptoms can range from mild respiratory disease to severe pneumonia with other affected organs that, in some patients, can induce death [1,2].
Initial treatment is symptomatic; oxygen therapy is the first step to improve respiratory symptoms. However, in those patients whose oxygen therapy does not lead to improvement, mechanical ventilation procedures are necessary, which requires transfer to intensive care units.
Different treatments have been used to control the infection and the evolution of the disease. However, poor effectivity was observed. This includes Convalescent Plasma treatment [3-6] or antiviral treatments such as Remdesivir, Ritonavir, Litonavir or Hydroxychloroquine [7-9]. To date, dexamethasone and monoclonal antibody therapies have shown partial benefits in patients with severe illness [10], while benefit from other drugs remains unknown [11].
The solubility of this alkaline hypertonic ibuprofenate (AHI) also retains the classical anti-inflammatory properties of ibuprofen, the non-selective and reversible inhibitor of cyclooxygenase (COX1 and COX2) that reduces the production of prostaglandins GE2, PGD2, PGF2α, PGI2) and platelet thromboxane A2, a vasoconstrictor that is pro-platelet aggregating and pro-inflammatory. Ibuprofen also shows anti-inflammatory activity through inhibition of neutrophil recruitment, activation and generation of reactive oxygen species [12]. Ibuprofen has been demonstrated to prevent inflammation in acute [13] and chronic [14] lung injury. These properties of Ibuprofen make its application feasible to control the "cytokine storm" induced by COVID-19 infection.
The objective of this retrospective statistical study was to analyze the changes observed in certain parameters in COVID-19 positive patients hospitalized at Orías Hospital in General San Martín, province of Jujuy, Argentina, treated with and without a nebulisable solution of AHI during two different periods. In the case of the control group (conventional treatment), data were taken from patients hospitalized between June and July, 2020; for those treated with AHI, data correspond to patients hospitalized between August and September, 2020.
This type of design is similar to that reported in a recent study comparing conventional treatment with colchicine therapy [15].
Methods
All the procedures followed in this study were in accordance with the ethical standards of the institutional and national guidelines.
The use of inhaled ibuprofen was carried out under the approval of the AHI formulation as compassionate treatment, extended by the Jujuy Ministry of Health, RESOLUTION No. 001651-S/2020.-EXPTE. No. 773-992/2020. In addition, the ethics committee of the Orias Hospital authorized the conduct of the study through Resolution 018-DH00/2020.
The control group of this study consisted of 62 SARS-CoV-2 RT-PCR positive patients admitted to Orías Hospital between June 11 and July 31, treated according to the protocols in force at that time, Dexa (8 mg/day) and Enoxa (5000 IU), supplemented with oxygen when needed. For the study group, the data collected corresponds to 37 patients infected with SARS-CoV-2 who were admitted to the Orías Hospital and who received treatment with AHI, in addition to basic therapy (Dexa - Enoxa) between August 3 and September 2.
It should be noted that physicians used the same clinical criteria to determine hospitalization for all patients. Symptoms and progression of the disease were followed in both groups, with and without AHI treatment.
Química Luar S.R.L. (Cordoba Argentina) provided the AHI used on a compassionate basis in hospitalized COVID-19 patients. In all cases, SARS CoV-2 infection was confirmed by PCR. Treatment consisted of administering, three times a day, an AHI solution (50 mg) by nebulization until oxygen saturation reached > 93% while breathing room air. Personal protective equipment was used for healthcare workers; treated patients wore a device designed to decrease the risk of contamination by aerosol.
A compassionate use program usually has no comparator therapy; however, in this case, we used the data provided by the hospital’s ethics committee for those patients hospitalized in Orías Hospital before administering Ibuprofen, as a comparator model to evaluate the effect of alkaline hypertonic Ibuprofen.
This report is based on data from patients who received only Dexa (6 mg/day) and Enoxa (5,000 Ui /24 h or, in few cases, each 12 h); oxygen was administrated when considered necessary during the period from Jun 11 through July 31, 2020. Data from patients who received the same treatment plus a supplemented compassionate treatment with AHI were obtained from August 3 through September 2.
Exclusion criteria included inability to use a nebulizer with a mouthpiece, allergy to ibuprofen, pregnancy, intention to become pregnant and breastfeeding.
The treatment consisted of 3 nebulisations daily with 5 ml of AHI for at least 7 to 10 days, with post-treatment follow-up for a maximum of 15 days.
Patients were required to understand the information provided in the consenting process and give consent.
Nebulization procedure
Data collection was carried out in all patients when admitted to hospital to generate their medical history and demographic data. Patients’ vital signs such as heart rate, respiratory rate, blood pressure, temperature and oxygen saturation were evaluated. In addition, a physical examination with clinical assessment for pneumonia was conducted.
For those patients hospitalized, 5 ml of alkaline hypertonic ibuprofen (AHI) containing 10 mg/mL of Ibuprofen in 0,5M NaCl with pH 8.5 was administered in a piston nebulizer (Silfab S.A., Argentina) three times a day for at least 7 days or, in some cases, during 10 days. While patients remained in hospital, we recorded their vital signs and the potential presence of adverse events/effects related to or produced by nebulization with AHI formulation.
Patient Involvement statement
To carry out this retrospective study, all patients provided informed written or verbal consent.
Results
Table 1 shows demographic and baseline characteristics, including comorbidities and severity of the disease. The mean age of the 62 patients without receiving ibuprofen treatment was 54.3 (SD 20.0): 45 (72.6%) were men and 17 (27.4%) women. The mean age of the 37 patients treated with AHI was 56.3 (SD 14.2): 27 (73%) were men and 10 (27%) women. It should be noted that around 60% of the patients were white; the remaining 40% corresponded to the native population of the "Guaraní" ethnic group.
Table 1:Demographic and baseline characteristics, including comorbidities and severity of the disease.
| Without AHI | With AHI | |
|---|---|---|
| Age | 54.3 (SD 20.0) | 56.3 (SD 14.2) |
| Sex | ||
| Men | 45 (72.6%) | 27 (73 %) |
| Women | 17 (27.4%) | 10 (27% ) |
| Ethnicity | ||
| White | 60% | 60% |
| Native population “Guaraní” | 40% | 40% |
| Comorbidities | ||
| All | 44 (70,97%) | 24 (64.86%) |
| Hypertension | 24 (38.71 %) | 18 (48.64%) |
| Heart disease | 0 (0%) | 1 (2.70%) |
| Diabetes | 7 (11.29%) | 9 (24.32 %) |
| Chronic lung conditions | 1 (1.61%) | 1 (2.70%) |
| Smoking | 2 (3.22%) | 0 (0%) |
| Severity of disease at baseline* | ||
| Isolated with no limitation in activities | 0 | |
| Isolated with limitation in activities | 1 | |
| Hospitalized patients | ||
| with mild illness (no oxygen therapy) | 2 | |
| with mild illness (oxygen therapy) | 11 | |
| with moderate disease (highflow) oxygen | 20 | |
| Mortality | (n=62) | (n=37) |
| Death | 24 (38.71%) | 3 (8.11%) |
| No death | 38 (61.29%) | 34 (91.89%) |
| Total | 62 (100.0%) | 37 (100.0%) |
| Odds Ratio | 8.427350427 | |
Of the 62 patients of the control group, 44 (64.7%) presented the same baseline comorbidities: diabetes, cardiovascular disease, hypertension, smoking habits. Here, 7 (10.29%) of the patients were diabetic and 16 (23.5%) were observed.
Data analysis from Table 1 shows that there is no statistically significant difference between the two groups in any of the parameters measured, such as age and sex (Table 2).
Table 2:Inferential statistical analysis per age and sex
| Parameters | Group 1 (Without AHI) | Group 2 (With AHI) | p-value | ||
|---|---|---|---|---|---|
| Media | SD | Media | SD | ||
| Age | 54.3 | 20 | 56.3 | 14.2 | 0.54693 |
| Gender Men | Percentage 72.6% | Percentage 73.0% | 0.60195 | ||
| Gender Women | Percentage 27.4% | Percentage 27.0% | 0.39805 | ||
| In this bilateral Hypothesis Test, we used Standard Normal Distribution (Z) Confidence Level (CI) 0.99 | |||||
The most significant comorbidities in both groups, with or without AHI treatment, were: hypertension, 24 (38.71%) and 18 (48.64%); diabetes, 7 (11.29%) and 9 (24, 32%) and obesity, 16 (25.81%) and 5 (13.51%), respectively. The number of patients with comorbidities such as hypertension and diabetes was similar in both groups. Yet, obesity was three times higher in those patients without receiving AHI treatment. This difference would partly explain the more unfavorable clinical evolution in this group.
Table 3 shows the Respiratory Rate values when both groups of patients were hospitalized. It is observed that there were no significant differences between them, with mean values of 25.3 (SD2.5) and 26.3 (SD 3.3) respectively.
Table 3:Average of initial FR from data from Figure 1 in patients with and without AHI treatment
| Group 1 (n=37) | Group 2 (n=62) | |
|---|---|---|
| Initial FR | With AHI | Without AHI |
| Average | 26.3 | 25.3 |
| SD | 3.3 | 2.5 |
Then, we analyzed the evolution of RR throughout the disease only in those patients treated with AHI. Figure 1 and Table 4 show that, at the end of AHI treatment, patients clearly improved their RR.
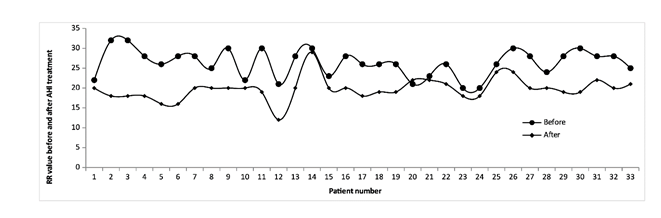
Figure 1:Respiratory rate of each patient treated with AHI at the beginning and end of hospitalization. The figure shows the data of the 33 patients who had been discharged upon completion of the trial. The remaining 4 patients of the total group of 37 remained hospitalized at that time.
Table 4:Inferential statistical summary of Respiratory Rate (RR) and O2 Saturation
| Parameter | Before AHI treatment | After AHI treatment | Valor p | ||
|---|---|---|---|---|---|
| Media | SD | Media | SD | ||
| RR | 26.3 | 3.3 | 19.8 | 2.9 | <0.00001 |
| One-sided Hypothesis Test, Standard Normal Distribution (Z) Confidence Level (CI) 0.99 | |||||
| Sat O2> | 88.3 | 4.3 | 95.8 | 2.7 | <0,00001 |
| One-sided Hypothesis Test, Standard Normal Distribution (Z) Confidence Level (CI) 0.99 | |||||
The analysis was performed using the one-sided Hypothesis Test with Standard Normal Distribution (Z) Confidence Level (CI) of 0.99. Results show that the mean RR of patients before receiving treatment with AHI was 26.3 (SD 3.3); after treatment, the mean was 19.8 (SD 2.9), with a value of p <0.00001. This represents a statistically significant RR reduction of 25%.
On the other hand, the analysis of oxygen saturation in patients treated with AHI is shown in the results of Figure 2 and Table 4.
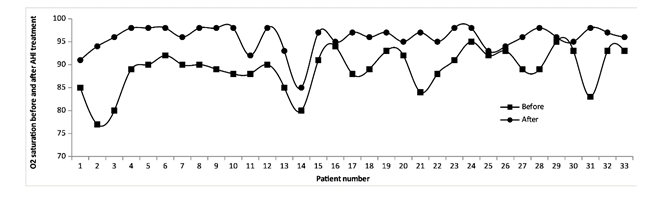
Figure 2:O2 saturation of each patient treated with AHI at the beginning and end of hospitalization. The figure shows the data of the 33 patients who had been discharged upon completion of the trial. The remaining 4 patients of the total group of 37 remained hospitalized at that time
The statistical analysis for O2 saturation was also performed with one-sided Hypothesis Test using Standard Normal Distribution (Z) Confidence Level (CI) of 0.99. The O2 saturation of patients before receiving treatment with AHI showed a mean of 88.3 with a SD 2.3. At the end of treatment, mean was 95.8 with a SD 2.7 with a p value <0.00001. These results show that AHI treatment leads to a statistically significant increase in O2 saturation. The O2 saturation data at the beginning and end of AHI treatment can also be seen in Table 4.
In those patients treated with AHI, discharge reached 85% in 14 days, while patients not treated with AHI, that is, patients who recovered with basic treatment, discharge only reached 36-37% at day 23.
These results show a steady/sustained recovery in the AHI-treated group, while patients who did not receive AHI show a late recovery that eventually reaches and overlaps the curve of the AHI-treated group.
Afterwards, we analyzed results related to deaths. Here we compared the death rate between patients treated without AHI (considered as control) and patients treated with AHI (problem). Treatment without AHI resulted in 24 deaths from 62 patients, representing 38.7% mortality. From the 37 patients treated with AHI, only 3 patients died, thus mortality was 8.1% (Table 5).
Table 5:Comparison of death rates (considering total number of patients in both sample groups)
| Mortality | With AHI (n=37) | Without AHI (n=62) |
|---|---|---|
| Death | 3 (8.1%) | 24 (38.7%) |
| No death | 34 (91.9) | 38 (61.3%) |
| Total | 37 (100.0%) | 62 (100.0%) |
| Odds Ratio | 8.427350427 |
To provide greater precision, the time of death was analyzed in relation to the length of hospitalization. Considering a time period of at least 5 days, Table 6 shows that in all AHI-treated patients who died, death ocurred within the first 10 days of hospitalization. In contrast, in patients not treated with AHI, deaths continued until day 25 of hospitalization.
Table 6:Summary Table. Statistical inference on the Percentage of Deaths according to the number of days of hospitalization
| Death (Percentage) | Without AHI | With AHI | Odds Ratio | P Value |
|---|---|---|---|---|
| 5 days or less | 16.2 | 2.7 | 6 | 0.019084445 |
| 10 days or less | 20.6 | 8.1 | 2.54 | 0.048613028 |
| 15 days or less | 27.9 | 8.1 | 3.44 | 0.008526679 |
| 20 days or less | 32.4 | 8.1 | 4 | 0.002664288 |
| 25 days or less | 33.8 | 8.1 | 4.17 | 0.005675704 |
Figure 4 shows a more detailed analysis of the relationship of AHI treatment with the number of deaths during daily follow-up. It is observed that deaths in patients treated with AHI mainly occurred from day 5 to day 9. This suggests that in those positive COVID-19 patients who tended to do poorly, treatment with AHI slowed its progression to a state of greater severity leading to death. On the other hand, in patients who did not receive AHI, death showed a sustained or constant growth that continued until day 25 of hospitalization.
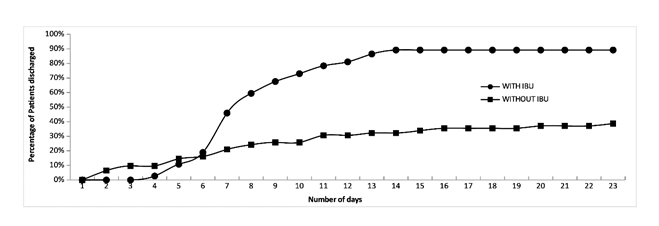
Figure 3:Percentage of patients discharged per day.
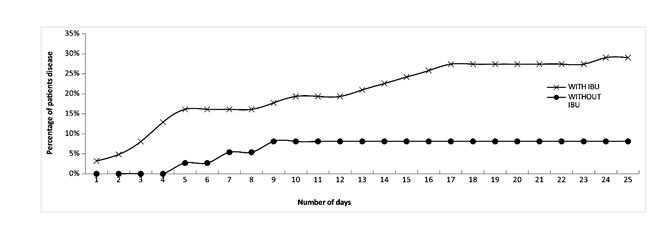
Figure 4:Percentage per day of patients treated with and without AHI who died (considering the total number of patients in both sample groups).
Discussion
We carried out a compassionate study with 37 patients hospitalized in Orías Hospital diagnosed with COVID-19 positive and treated with basic treatment comprising Dexa and Enoxa. However, they were also treated with a solution of alkaline hipertonic ibuprofen via nebulization. The study was conducted over one month (August 3 to September 2, 2020) to evaluate treatment safety and efficacy. The control group was made up of 68 COVID-19 positive patients admitted to the same hospital between June 11 and July 31. Both groups, control and problem, were assisted by the same medical personnel.
Both groups (treated with AHI and without AHI) were matched for sex, age and general comorbidities such as diabetes, heart disease and hypertension, chronic lung disease, and smoking habits. However, the number of obese patients was three times higher in the group of patients who did not receive AHI treatment. Obesity constitutes one of the most important risk factors reported in COVID-19.
Since COVID-19 is a pathology with a markedly inflammatory process, especially at the pulmonary level, the rationale for this route of administration is to enable maximal delivery of the active drug to the biological focus of SARS-CoV-2 infection, the lung (pulmonary epithelium).
The results of this compassionate pilot study have shown that inhaled AHI, given 3 times a day (each 8 h) by nebulization with a dose of 50 mg for 7 or 10 days, according to clinical criteria, is well tolerated and effective in improving the symptoms of SARS-Cov2 infection. The comparison of both groups shows that a greater proportion of patients treated with AHI could recover during the 10-day study period, more noticeably, after day 25.
In agreement with previous observations, nebulization with AHI showed no serious adverse symptoms. The two most common adverse effects found in people nebulized with AHI included mild irritation in the nose, mouth and throat, sometimes leading to cough. This could be due to the presence of the hypertonic saline concentration of 0.5 M NaCl.
In accordance with the report by Salva et.al. [16], both results suggest that AHI nebulization is safe.
In relation to the results on respiratory rate (RR) symptoms, the analysis revealed that AHI treatment produced a statistically significant improvement in symptoms, as compared to those patients not treated with AHI.
Results of oxygen saturation in patients from both groups (Figure 2 and Table 1) show that treatment with AHI significantly improves O2 saturation. Both positive effects could be associated with the properties of Ibuprofen, especially when directly applied to the lung. On the other hand, it is interesting to note that the AHI solution has an alkaline pH of 8.5.
Capellini et.al., working with rat aortic endothelial and smooth muscle cells, shows that when the extracellular pH reaches a value of 8.5, the same as the AHI solution, intracellular changes occur that lead to an increase in the synthesis and release of nitric oxide produced by the rapid activation of NO synthase. The release of NO from the endothelium produces rapid vasodilation that improves oxygenation and reduces pulmonary vascular resistance, promoting blood flow in the lung [17]. On the other hand, nitric oxide released by endothelial cells and platelets also prevents platelets from adhering to blood vessels, which helps remove microaggregates, inhibits platelet adhesion and aggregation, and prevents vascular occlusion [18].
In this sense, it has been shown that ibuprofen increases the activity of nitric oxide synthase (iNOS) and, in addition, shows a marked effect on circulating platelets [19-21]. As other authors have proposed for NO, the aforementioned antecedents justify considering ibuprofen as a therapeutic strategy in the management of patients with COVID-19 [22].
Currently, options for treating COVID-19 remain limited. Recent evidence of the use of dexamethasone indicates that it is less effective in those patients with COVID-19, who show less severe symptoms [10]. A recent "SOLIDARITY" report mentions similar results for the treatment of COVID-19 with inhaled interferon beta [23]. The authors report that treatment with nebulized inhaled interferon β 1a produces promising results. The effect observed after administration of interferon beta by inhalation is substantially safer than that requiring dangerously high doses intravenously.
Figure 5 shows the percentages of patients who were discharged in both groups, being 80% in those patients treated with AHI and only 36-37% in patients not treated with AHI (patients who healed alone or with the basic Dexa-Enoxa treatment). On the other hand, the analysis shows that a linear increase is seen from day 1 to day 15, then it slows down. In patients not treated with AHI, no discharge was observed until day 4, then, from day 5 to 8, a greater increase was found, reaching the curve of patients treated with AHI.
Finally, analysis of the total number of patients who died in both groups shows that 24 of the 62 patients without AHI died, which represents 38.7%. In the group of patients treated with AHI (37), only 3 died, with a mortality rate of 8.1%. This result represents a decrease of 79% in mortality in patients treated with Ibuprofen.
The Odds ratio to death between patients that use AHI versus patients without treatment with AHI shows a great value of 8.427350427. This means that patients who were nebulised with AHI possess 8 times less chance to die that those patients without nebulization with AHI.
The analysis of deaths as a function of time shows that in the group of patients with AHI treatment, deaths occurred during the first 4 days, and no deaths were recorded after day 5. In the group of patients not treated with AHI, an increasing mortality curve was observed from day 1 to day 25. This result suggests that AHI treatment, even in the most seriously ill patients, has a comparatively beneficial effect. In relation to the high mortality value observed in those patients not receiving treatment with AHI (control group), it should be noted that they were patients who were admitted to the hospital in June and July, that is, at the beginning of COVID-19 in Jujuy province, where no experience had been gained in treating the disease. In addition, these patients probably went into hospital too late, when symptoms were already evident, which could decrease the effectiveness of treatments. All this could explain the high mortality rate observed in this group.
Considering that COVID-19 is an inflammatory disease, Ibuprofen constitutes a valid alternative to treat the consequences of this disease. Ibuprofen is a widely known and used anti-inflammatory. These molecules non-selectively and reversibly inhibit cyclooxygenase (COX1 and COX2), which reduces the production of prostaglandins GE2, PGD2, PGF2α, PGI2) and platelet thromboxane A2 (a vasoconstrictor), inhibiting platelet aggregation.
One of the mechanisms put forward for the entry of viruses into cells are those mediated by Rho-GTPase regulated by actin. A recent work demonstrates that the ibuprofen R- enantiomer is a Rho-GTPase inhibitor (RhoA, Cdc42 and Rac1 are representative Rho-GTPases), and ibuprofen is a specific inhibitor of Rac1b expression [24]. It has also been reported that Ibuprofen may interact with actin molecule [25]. In other "in vitro" studies it was demonstrated that Ibuprofen shows bactericidal and virucidal activity [26,27]. Through computational studies, it was recently suggested that Ibuprofen could interact with M-pro, the major protease present in Coronavirus [28].
In vivo studies also demonstrated that ibuprofen produces a significant decrease of neutrophil adherence to pulmonary endothelium induced by serum. This protective effect may be due to a reduction of neutrophil sequestration in lung [29].
Onischuk also reports the results obtained from the evaluation of the anti-inflammatory and analgesic effect of ibuprofen through the Relative Analgesic Index (RAI) [30,31]. In this assay, ibuprofen was administrated to mice orally and via nebulization. The aerosol treatment of ibuprofen is approximately four orders of magnitude (10,000 times) more effective than that administered orally.
Pulmonary route and not oral administration was the best method to obtain greater biological activity. Taken together, the results of this comparative compassionate study suggest a potential clinical benefit of nebulizable AHI to treat patients admitted to hospital with COVID-19. Phase 2 clinical trials. A higher number of patients and further predefined conditions of placebo and problem groups will allow a more in-depth evaluation of the results of this treatment.
Funding Information
This research was supported by Química Luar SRL and CEPROCOR, no specific grant was received from any funding entity.
Declaration of Interests
There are no conflicts of interest of the participants of this work to declare.
Contributorship Statement
Galia Kalayan coordinated the development and data collection; Ana Carolina Arias Cau, Manuela del Valle Cabello, Mariela Nuñez, Romina Lumetto were the ones who collected the data and made their selection; Dante Beltramo, Roxana Alasino, Luis Argañaras, Néstor García and Nicolás Martinez Rios had the idea for the article; Dante Beltramo and Roxana Alasino wrote the article.
References
1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061-1069. doi:10.1001/jama. 2020.1585
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323 (13):1239-1242. doi:10.1001/jama.2020.2648
3. Ling Li, Wei Zhang, Yu Hu, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19 - A Randomized Clinical Trial. JAMA. 2020; doi:10.1001/jama.2020.10044
4. Simonovich VA, Burgos Pratx LD, Scibona P, et.al. PlasmAr Study Group. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2020; doi: 10.1056/NEJMoa2031304. Epub ahead of print. PMID: 33232588.
5. Libster R, Pérez Marc G, Wappner D, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021; 384:610-618. doi: 10.1056/NEJMoa2033700.
6. The RECOVERY Collaborative Group, Horby PH, Estcourt L, et.al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021.03.09.21252736;
7. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19-Preliminary Report. New Engl J Med 2020; doi:10.1056/NEJMoa2007764.
8. Cao B, Wnag Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020; 382:1787-99 doi: 10.1056/NEJMoa2001282
9. Cavalcanti AB, Zampieri FG, Rosa RG, et.al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. New Engl J Med. 2020; doi: 10.1056/NEJMoa2019014 (update with details)
10. RECOVERY Collaborative Group, Horby P, Lim WS, et.al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021; 384(8):693-704. doi: 10.1056/NEJMoa2021436. Epub 2020 Jul 17. PMID: 32678530; PMCID: PMC7383595.
11. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J. Med. 2020; doi: 10.1056/NEJMoa2023184. Epub ahead of print. PMID: 33264556.
12. Villanueva M, Heckenberger R, Strobach H, et al. Equipotent inhibition by R(-), S(+) and racemic ibuprofen of human polymorphonuclear cell function in vivo. Br J Clin Pharmacol 1993 35:235-242.
13. Huang LT, Lin CH, Chou HC, et al. Ibuprofen protects ventilator-induced lung injury by downregulating Rho-kinase activity in rats. BioMed Res Int. 2014; doi:10.1155/2014/749097.
14. Elizur A, Cannon CL, Ferkol TW. Airway inflammation in Cystic Fibrosis. Chest. 2008; 133(2)489-95. doi: 10.1378/chest.07-1631.
15. Landray M. RECOVERY (Randomised Evaluation of COVID-19 therapy) Colchicine arm stopped for lack of efficacy in patients hospitalised with COVID-19.https://t.co/hcn9PytegJ pic.twitter.com/q09eddYXYL
16. Oscar Salva , Pablo A Doreski, et al. Rersal of SARS-CoV2-Induced Hypoxia by Nebulized Sodium Ibuprofenate in a Compassionate Use Program. Infect Dis Ther. 2021. doi: 10.1007/s40121-021-00527-2.
17. Capellini VK, Restini CBA, Bendhack LM, et al. The Effect of Extracellular pH Changes on Intracellular pH and Nitric Oxide Concentration in Endothelial and Smooth Muscle Cells from Rat Aorta. PlosOne 2013 doi:10.1371/journal.pone.0062887.
18. Freedman J.E, Loscalzo J. Nitric oxide and its relationship to thrombotic disorders. - Journal of Thrombosis and Haemostasis 2003, 1: 1183–1188.
19. Jiménez D, Martin MJ, Pozo D, et al. Mechanisms involved in protection afforded by L-arginine in ibuprofen-induced gastric damage: role of nitric oxide and prostaglandins. Dig Dis Sci. 2002;47(1):44-53. doi: 10.1023/a:1013203217788. PMID: 11837731.
20. Menzel JE, Kolarz G. Modulation of nitric oxide synthase activity by ibuprofen. Inflammation. 1997;21(4):451-61. doi: 10.1023/a:1027374605731. PMID: 9276767.
21. Salva Oscar, Alasino Roxana, Giller Celia, et al. Nebulization with alkaline hipertonic ibuprofen induces a rapid increase in platelets circulating in COVID-19 patients but not in healthy subjects. Platelets. 2021; 1-8 doi: 10.1080/09537104.2021.1967918.- 2021.
22. Fang W, Jiang J, Su L, et al. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic Biol Med. 2021;163:153-162.
23. Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial . Lancet Respir Med. 2020; doi: 10.1016/ S2213-2600(20)30511-7.
24. Li R, Song X, Li G, et al. Ibuprofen attenuates interleukin-1β-induced inflammation and actin reorganization via modulation of RhoA signaling in rabbit chondrocytes - Acta Biochim Biophys Sin (Shanghai). 2019;51(10):1026-1033. doi: 10.1093/abbs/gmz101.
25. Veljkovic V, Vergara-Alert J, Egalés J, et al. Use of the informational spectrum methodology for rapid biological analysis of the novel coronavirus 2019-nCoV: prediction of potential receptor, natural reservoir, tropism and therapeutic/vaccine target. 2020; 9:52.
26. Muñoz AJ, Alasino RV, Garro AG, et al. High Concentrations of Sodium Chloride Improve Microbicidal Activity of Ibuprofen against Common Cystic Fibrosis Pathogens. Pharmaceuticals. 2018; 11-47.
27. García NH, Porta DJ, Alasino RV, et al. Ibuprofen, a traditional drug that may impact the course of COVID-19 New effective formulation in nebulizable solution. Medical Hypotheses 2020; 144: 110079.
28. Clemente CM, Freiberger MI, Ravetti S, et al. An in silico analysis of Ibuprofen enantiomers in high concentrations of sodium chloride with SARS-CoV-2 main protease. J Biomo Structure & Dynamics. 2021; doi: 10.1080/07391102.2021.1872420.
29. Perlman MB, Johnson A, Malik AB. Ibuprofen prevents thrombin-induced lung vascular injury: mechanism of effect. The Am Physiol Soc. www.physiology.org/journal/ajpheart at Midwestern Univ Lib (132.174.254.157) 2019.
30. Onischuk AA, Tolstikova TG, Sorokina IV, et al. Analgesic Effect from Ibuprofen Nanoparticles Inhaled by Male Mice. J Aerosol Med Pulmonary Drug Delivery. 2009 doi:10.1089/jamp.2008.0721
31. Onischuk AA, Tolstikova TG, An´kov SV, et al. Ibuprofen, Indomethacin and Diclofenac Sodium Nanoaerosol: Generation, Inhalation Delivery and Biological Effects in Mice and Rats. Journal of Aerosol Science 2016. Doi: 10.1016/j.jaerosci.2016.05.005.
Received: April 01, 2022;
Accepted: April 25, 2022;
Published: April 28, 2022.
To cite this article : Kalayan G, Cau ACA, Cabello MDV, et al. Safety and Efficacy of Nebulised Anti-Inflammatory Solution of Alkaline Hypertonic Ibuprofen (AHI) for Treatment of SARS-Cov-2 Infection: A Compassionate Study with a Comparator Arm. European Journal of Respiratory Medicine. 2022; 4(2): 313 -319. doi: 10.31488/EJRM.132.
© 2022 Kalayan G, et al.
