Research Article / Open Access
DOI: 10.31488/ejrm.107
Safety and Efficacy of an Innovative Airway Clearance Device Versus Manual Chest Physiotherapy Techniques For Airway Secretion Clearance: a Feasibility Study
Philippe Giovannetti*1, Laurent Morin*2, Martine Reynaud-Gaubert*3
1.Service de Pneumologie, Centre de Ressource et de Compétence de la Mucoviscidose (CRCM) adulte, Centre de Compétence national des Hypertensions Pulmonaires (HTAP) et des Maladies Pulmonaires Rares, APHM-CHU Hôpital Nord, Marseille, France
2.Physio-Assist, Aix-en-Provence, France
*Corresponding author: Dr Laurent MORIN, Physio-Assist, 31 Parc du Golf, CS 90519, 13593 Aix-en-Provence, France.
Abstract
Background:Airway clearance therapy is an important part of treatment strategies in patients with respiratory disease and impaired mucociliary transport. This feasibility study evaluated the risks and benefits of a new airway clearancedevice (ACD) compared with manual chest physiotherapy (CP) in patients with obstructive lung diseases. Materials and Methods:A prospectivetrial was conducted at a pulmonology center in Marseille, France. 15 patients (cystic fibrosis, bronchiectasis, COPD) with stable respiratory function receivedpersonalized CP sessionsduring 4 days. Two morning sessions with manual CP(days 2 and 4) were replaced by the ACD (Simeox; Physio Assist). Patients received only CP sessions on days 1 and 3. Side effects, volume of sputum, oxygen saturation, duration of treatment, and patient acceptance/preference were compared between device and CP sessions. Results:All vital signs remained stable and there was no decrease in oxygen saturation during device usage. One pneumothorax occurred, but this was not considered to be CP or device related. Device therapy was comfortable and painless in 90% of patients, although some patients with very impaired lung function found the procedure quite tiring. 24h-weight of sputum was higher after the first device (41.122.1g) versus the first CPsession (27.515.6g). 80% of patients were satisfied with the effectiveness of ACD, and most (73%) preferred sessions with the device. Conclusions:Short-term use of the Simeox ACD had a good safety profile and was effective. The results of this preliminary study require validation in a larger cohort of patients with obstructive lung disease and chronic mucus hypersecretion.
Keywords: airway clearancetechnique,obstructive lung diseases,chest physiotherapy,cystic fibrosis
Introduction
Airway clearance therapy is an important treatment strategy in patients with respiratory disease and impaired mucociliary transport (e.g. cystic fibrosis [CF])[1-4].Airway clearance techniques (ACTs) supplement the mucociliary clearance system in the presence of disease-related impairment[2], and are important for maintaining respiratory health [4]. Current airway clearance interventions are based mainly on physical or mechanical measures to move air behind mucosal obstruction, and modulation of expiratory airflow to enhance movement of secretions to more proximal airways for more effective clearance[2].
ACTs in obstructive lung diseases encompasses very various interventions including1/ manual chest physiotherapy (CP): conventional CP (postural drainage, percussion, vibration, forced expiration technique, directed cough), autogenic drainage and active cycle breathing technique, and 2/ airway clearance devices (ACD) as Positive Expiratory Pressure (PEP), oscillating PEP andhigh frequency chest wall oscillations[2, 5].ACTs have been shown to have beneficial short-term effects in increasing transport mucus away [5, 11] with no clear differences in effectiveness between techniques[6-10,12,22].
Whatever the approach, the overall goal of airway clearance therapy is to improve mucus clearance, thus decreasing the risk of bronchial infection, attenuating disease progression and lung function decline, and improving quality of life[4, 11-13]. However, strategies such as CP are intensive and time-consuming, may require assistance from a physiotherapist or caregiver, and can be uncomfortable for patients[5].Furthermore, these strategies require time and effort from the patient and their caregivers, and adherence to CP may be suboptimal in adults with CF[14],and compliance with CP is comparatively low in this group (53%)[15].
Use of medical devices offers an additive or alternative airway clearance strategy with similar effectiveness to CP[6, 7, 9, 10, 12, 16, 17, 22]. This feasibility study investigated the risks and benefits of a new ACD, a pneumatic vibratory stimulus device (Simeox; PhysioAssist, France) in patients with obstructive respiratory diseases and chronic mucus hypersecretion.
Materials and Methods
The SIMETOL trial (NCT02061852) was a prospective, non-randomized, single center, open-label, sequential, cohort study conducted at an adult pulmonology center in Marseille, France from 21 July 2014 to 24 September 2015. The study protocol was approved by the CPP Sud-Méditerranée I ethics committee in Marseille, France (Ref: 2014-A00079-38). All patients provided written informed consent prior to enrolment in the study. The study was conducted in accordance with Declaration of Helsinki principles and good clinical practice guidelines, all applicable rules governing medical devices, and any local regulations.
Patient population
Eligible outpatients were aged 18 years and met the following inclusion criteria: chronic obstructive pulmonary disease (COPD) with bronchorrhea; chronic bronchiectatic disease, CF, primary ciliary dyskinesia and/or chronic bronchitis with sputum; predictable hospitalization duration of 5 consecutive days; bronchial clearance that was usually productive;forced vital capacity (FVC) and/or forced expiratory volume in 1 second (FEV1) <85% of predicted, with stable respiratory function; agreed to study participation and provided informed consent.
Patients were excluded from the study if they experienced bronchial infection with pan-antibiotic-resistant bacteria, had a contraindication for airway clearance therapy, required more than two chest physiotherapy (CP) sessions each day, had undergone previous lung transplantation, required mechanical non-invasive ventilation for more than eight hours per day, had history of hemoptysis or pneumothorax in the last 12 months, were pregnant or breastfeeding, had participated in another clinical trial within the previous 30 days, were unable or unwilling to adhere to the study protocol, or had any other condition that precluded enrolment into the study in the opinion of the investigator.
Objectives
The objectiveswere to investigate the safety of the ACD, including vital signs, tiredness, and breathing discomfort, effectiveness of bronchial drainage based on the quantity of sputum over a 24-hour period (primary performance criteria), session duration, and patient evaluation and preference.
Interventions and medical device
The Simeoxmedical device used in this study (Figure 1) applies a precise pneumatic vibratory stimulus to the bronchial tree during relaxed exhalation by disseminating a succession of intermittent negative air pressure pulses at different frequencies (6 and 12 Hz).
Figure 1. SimeoxAirway clearance device
The pneumatic signal exerts shearing forces on mucus causing it to liquefy, and suction occurs via negative pressure. Between signal pulses, atmospheric pressure returns and the sputum returns to the solid phase, preventing it from going back down the airway. As a result, mucus is progressively mobilized along the bronchi to more proximal airways for productive expectoration.
The device consists of a turbine generating negative air pressure, a vibration generator, a microcontroller controlling vibration frequency and all user interfaces, and a data display interface and PC dialogue for analysis of results. This is connected to a breathing system that includes a mouthpiece, a protection filter for the single-use breathing chain, a flexible tube, and a machine protection filter. During the study, the device was controlled by a touchscreen application on a tablet, which allowed the frequency and delivered power to be varied, and visualized the negative pressure generated by the device and the patient. One treatment session with the device consisted of four series of ten respiratory cycles with a 1-minute rest between series (total of 40 respiratory cycles depending on patient tolerance).
To ensure the study was performed in a controlled environment, patients were treated in hospital for 4 days according to usual clinical practice. On days 1 and 3 the morning treatment session consisted of manual CP, and on days 2 and 4 the morning session was performed withthe ACD; a manualCP session was also performedin the afternoon of all four days if there was persistent bronchial congestion (Figure 2).
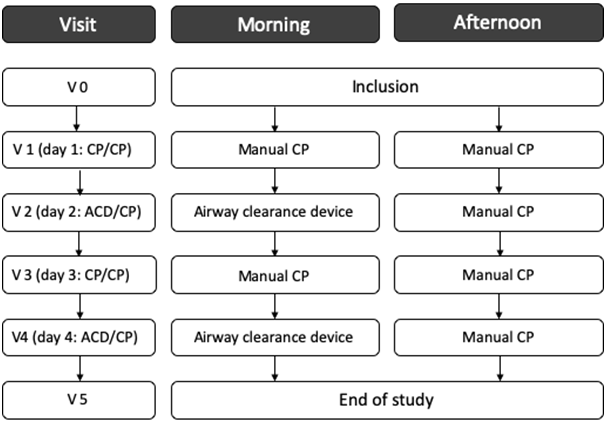
Figure 2. Flow chart of study visits
Assessments and follow-up
Total study duration was 5–8 days, depending on the duration of patient hospitalization (all visits had to be completed within 8 days of visit 1). The duration of each session (minutes) was recorded by the chest physiotherapist.
Adverse events (AEs) were reviewed by the physician investigator, chest physiotherapist and sponsor. The following information was collected for all AEs: description; nature; start/end dates; severity; seriousness; evolution/outcome; causal relationship with the device; and interventions required. Patients were questioned about tolerability (tiredness and breathing discomfort, both rated on a 4-point scale from 0 (not at all tiring/painful) to 3 (very tiring/painful). Oxygen saturation (SpO2) was measured before and after each treatment session using a pulse oximeter (Nonin Medical Inc., USA), and vital signs were determined at each study visit.Any issues related to the identity, quality, durability, reliability, safety or performance of the device were reported by the patient and/or chest physiotherapist.
Average weight of 24-hour sputum collected from the start of one morning session to before the next morning session was calculated. Patients were asked to rate their satisfaction with therapy and their therapy preference (device, device + CP, or CP). Patient satisfaction was determined after each session by asking patients the following question: “The efficiency of Simeox session seems to you very satisfactory (score +2) OR fairly satisfactory (score +1) OR unsatisfactory (score –1) OR not at all satisfactory (score –2)?”.
Statistical analysis
The intention-to-treat (ITT) population included all enrolled and treated patients. Missing data were not included in the denominator for calculation of percentages.
Descriptive analyses (mean, standard deviation [SD], median, interquartile andrange for quantitative variables; and frequency and percentage for qualitative variables) were used to report baseline patient characteristics, vital signs, AEs and device deficiencies. The occurrence of AEs in the device and CP groups was compared using Fisher test or Chi-2 test. Quantitative data were compared within groups using the paired Student t-test or paired non-parametric tests (Wilcoxon signed rank test) based on normality of distribution. Patient preference and satisfaction scores were calculated and compared with a null reference score.
All analyses were performed using R software for Windows. A p-value < 0.05 was considered statistically significant.
Results
A total of fifteeneligible patients were included in the study. All patients completed 4 days of therapy and were evaluated for ITT analysis. The most common respiratory condition was CF, and concomitant medications were consistent with reported medical histories (Table 1).
Table 1 Patient characteristics at baseline (intention-to-treat population)
| Patients (n=15) | |
|---|---|
| Male, n (%) | 10 (66.7) |
| Age, years | 37.0±10.6 (19.0–54.4) |
| Body mass index, kg/m2 | 19.9±2.9 (15.2–26.1) |
| Systolic blood pressure1, mmHg | 116.5±18.0 (90.0–145.0) |
| Diastolic blood pressure1, mmHg | 75.3±11.5 (60.0–96.0) |
| Heart rate1, beats/min | 75.3±11.5 (60.0–96.0) |
| Respiratory disease, n (%) | |
| COPD | 1 (6.7) |
| Chronic bronchiectatic disease | 2 (13.3) |
| Cystic fibrosis | 11 (73.3) |
| Idiopathic bronchiectasis | 1 (6.7) |
| Current non-invasive ventilation, n (%) | 8 (53.3) |
| Duration of non-invasive ventilation, h/day | 6.3±2.1 (4.0–8.0) |
| Current oxygen therapy, n (%) | 3 (20.0) |
| Disease history, n (%) | |
| Hemoptysis | 6 (40.0) |
| Pneumothorax | 1 (6.7) |
| Chronic bronchial colonization, n (%) | 15 (100.0) |
| Pseudomonas aeruginosa | 13 (86.7) |
| Staphylococcus aureus | 5 (33.3) |
| Aspergillus fumigatus | 2 (13.3) |
| other | 5 (33.3) |
| Intravenous antibiotic cures in the previous year4, n | 2.9±2.0 (0.0–5.0) |
| Hospitalizations in the previous year4, n | 1.3±1.2 (0.0–4.0) |
| Physiotherapy sessions5, number/week in the last month | 2.4±3.0 (0.0–8.0) |
| Self-drainage sessions4, number/week in the last month | 5.3±4.1 (0.0–14.0) |
| Pulmonary function2 | |
| FVC, % predicted | 51.3±14.5 (34.1–82.4) |
| FEV1, % predicted | 29.1±9.5 (15.3–45.9) |
| FEV1/FVC ratio, % | 51.1±6.3 (40.8–62.2) |
| Blood gases3 | |
| PaO2, mmHg | 71.1±13.0 (53.0–94.0) |
| PaCO2, mmHg | 40.2±5.1 (32.0–51.0) |
| pH | 7.4±0.0 (7.3–7.5) |
| SaO2, % | 93.5±4.1 (85.0–99.0) |
Values are mean standard deviation (range), or number of patients (%).
COPD, chronic obstructive pulmonary disease; FEV1, force expiratory volume in 1 second; FVC, forced vital capacity; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; Q, quartile; SaO2, oxygen saturation; SD, standard deviation.
1Blood pressure and heart rate were measured in the sitting position after a 5-minute rest period; blood pressure data were missing for 4 patients and heart rate data for 3 patients.
2Data were missing for 1 patient for FVC and 2 patients each for FEV1 and FEV1/FVC ratio.
3Data were missing for 1 patient for PaCO2 and 2 patients each for PaO2 and pH
4Data missing for 6 patients.
5Data missing for 3 patients.
Mean SD study duration was 6.91.1 days (range 4–8 days). The majority of patients performed the afternoon manualCP session (n=10 at visit 1, n=15 at visit 2, n=11 at visit 3, and n=13 at visit 4; p=0.11). The main airway clearance technique used during manual CP sessions was autogenic drainage (60–100% of sessions); other techniques included forced expiration (7–40% of sessions) and directed cough (7–20% of sessions). Session duration was similar for deviceand CP (30.39.3 vs. 27.34.1 minutes [p=0.24], respectively).
Safety
All vital signs were stable throughout the study. The only AE was a serious event (left-sided pneumothorax [2 cm] with moderate nocturnal pain and no other signs of seriousness in a CF patient colonized with Pseudomonas aeruginosa and Staphylococcus aureus who had received five antibiotic treatments in the previous 12 months), and no previous history of pneumothorax. The pneumothorax event occurred between visit 3 (CP/CP) and visit 4 (device/CP), and was not considered by investigators to be related to CP or the device. Treatment consisted of pleural drainage and resolved without sequelae within 5 days.
Patients found device therapy tiring, with ratings of ‘little tiring’, ‘quite tiring’ and ‘very tiring’ in 27%, 40% and 13% of patients, respectively (p<0.001 for mean score vs. null value; ITT). One patient temporally discontinued device therapy for tiredness during the first session but performed the second device session well. Patients who rated the device sessions as tiring had significantly worse lung function than those who had mild or no tiredness (FEV1pred.: 23.1±4.6 vs. 37.2±8.3 %; p=0.003) and FVC pred.: 42.2±5.6 vs. 63.4±14.0 %; p=0.003, respectively).
Device therapy was rated as ‘not at all painful’ (67%) or ‘little painful’ (20%; p=0.053 for mean score vs. null value); none rated device therapy as ‘very painful’. One patient stopped therapy for nausea during the last cycle of the second session.
Mucus clearance and lung function
The average weight of 24-hour sputum was slightly, but not significantly, higher in sessions that included use of the device (device/CP; days 2 and 4) versus CP alone (CP/CP; days 1 and 3) (Table 2).
| Device/CP | CP/CP | p-value(paired t-test) | |
|---|---|---|---|
| Weight of 24-hour expectorated sputum per patient, g | 33.3±18.0 | 29.3±15.0 | |
| Intention-to-treat population(n=12)1 | (36.8; 17.2-48.9) | (28.5; 16.8-35.1) | 0.28 |
Values are mean ± standard deviation (median; IQR)
1Data missing for 3 patients.
CP, chest physiotherapy
Median weight of 24-hour sputum was 29% higher in the device versus CP group. The greatest volume of sputum was achieved after the first device session (visit 2 vs. visit 1: 41.122.1vs. 27.515.6g) (Figure 3). SpO2did not decrease during device use and remained stable during the study. However, there was less negative SpO2variation from before to after each day’s sessions includingdevice (device/CP; days 2 and 4)versus CP alone (CP/CP; days 1 and 3) (Figure 4), and when only morning sessions (device or CP) were compared (Figure 5).
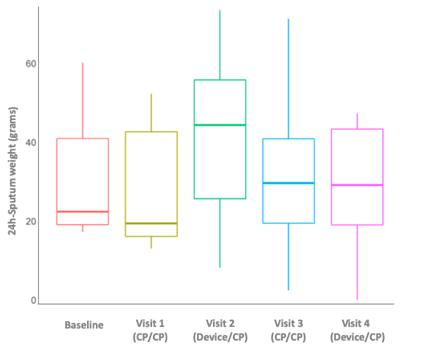
Figure 3. Twenty-four-hour expectorated pulmonary sputum by study visit (intention-to-treat population). CP, chest physiotherapy.
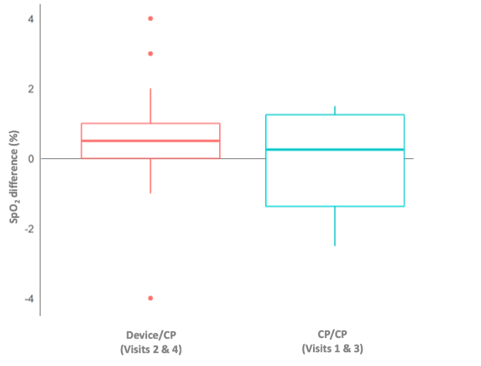
Figure 4. Average oxygen saturation (SpO2) difference from before morning therapy sessions to after afternoon therapy sessions (intention-to-treat population). CP, chest physiotherapy.
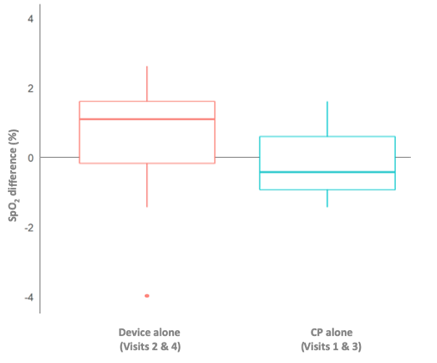
Figure 5. Average oxygen saturation (SpO2) difference from before to after morning therapy sessions (intention-to-treat population). CP, chest physiotherapy.
Patient satisfaction/preference
The proportion of patients who were ‘very’ or ‘fairly’ satisfied with the device therapy was 80%; comparison of the mean satisfaction score (0.6) with the null scoreapproached statistical significance (p=0.06). Seventy-three percent of patients reported a preference for device-only or device + CP session; the remaining patients (27%) preferred CP alone.
Discussion
This study provides the first clinical data on the Simeox airway clearance device. There is currently no equivalent medical device available. Due to the novelty of the device, this preliminary investigation was conducted in a hospital setting with monitoring by a physician and chest physiotherapist and the primary objective was safety, although ultimately the device may be able to be used at home by patients. The results showed that device therapy had an acceptable safety profile.
The mean age and body mass index, low FEV1 % pred. and FEV1/FVC ratio reflects disease severity in the general population with poor lung function and nutritional status, especially those with CF (73% of the study population)[8, 11, 18, 19]. This predominance of CF patients in our study reflects the target population likely to use the medical device under investigation.
No side effects were identified during the study. There was only one AE during the trial (pneumothorax), which occurred after the second CP session and before the second device session in a patient with CF and was not considered to have a causal relationship with the ACD being studied for a number of reasons. Firstly, there was no close temporal relationship between device use and the pneumothorax event. In addition, pneumothorax is known to be a serious complication in CF[20], and is more common in older patients and those infected with P. aeruginosa, Burkholderiacepacia or Aspergillus[21], as was the case in our patient. Furthermore, the patient who experienced pneumothorax was receiving two inhaled treatments, and use of inhaled medications has also been associated with pneumothorax[21]. Therefore, the likelihood thatdevice therapy contributed to the development of pneumothorax is considered to be very low.
Although not always reported in the literature, the risk of AEs when using non-pharmacological management strategies in respiratory disease appears to be relatively small[8, 22]. However, some physiotherapy techniques (e.g. postural drainage with clapping or active cycle breathing) have been shown to decrease SpO2[6, 10]. In contrast, we observed less negative variation in SpO2 with the device versus CP. Furthermore, there did not appear to be any additive risks when combining ACD with manual CP in the current study.
Volume or weight of expectorated sputum has been used as an endpoint in previous studies[4, 11, 23]. In our study, median cleared sputum volume tended to be higher when the device was used. However, between-group differences failed to reach statistical significance, possibly due to the small sample size. Nevertheless, the results consistently indicated a trend toward better sputum expectoration when the ACD was used during physiotherapy session. The greatest sputum clearance volume was achieved after the first session that included device usage (Figure 3). Thus, initial device use could have mobilized secretions that had not been cleared by previous CP. The device may therefore be a useful adjunct to CP, facilitating access to different and deeper lung areas, with benefits from the first use.
Patients perceived that the addition of the ACD to CP had a beneficial effect. Some patients did experience tiredness during treatment sessions with the device, but those who found the sessions tiring had very impaired lung function meaning that their endurance and strength was reduced to start with. Importantly, patients did not consider device therapy to be painful, and this may have contributed to the good satisfaction ratings. These results suggest that drainage session can be performed even in patients with very severe disease while taking some precautions to avoid excessive effort of the patients during the procedure. The finding that most patients were satisfied with device therapy is important because patient satisfaction has been associated with better treatment adherence and a resulting reduction in infection risk[2, 4, 23, 24]. Session duration was similar between both therapies, indicating that device use does not increase patient burden in terms of time required for treatment.
It has been suggested that patients with CF may prefer self-administered airway clearance treatments over manual CP, even if effectiveness is similar[23]. The optimal strategy for an individual patient may include more than one technique[2], and in this study the addition of the device to CPwas favored by two-thirds of patients. Furthermore, although the quality of evidence for the use of airway clearance techniques in CF has been described as ‘fair’ and the benefits ‘moderate’[4], guidelines state that patient preference should be considered when choosing therapies, with the hope that this would improve adherence to therapy[23]. Indeed, patient preference has been shown to be an important determinant of adherence to airway clearance therapies[25].
The main limitation of this preliminary investigation is the small sample size, which might have contributed to a lack of statistical significance for some between-treatment comparisons. However, the primary objective was to evaluate device safety in patients with obstructive lung disease and this was achieved. Other limitations were the lack of a separate control group (each patient acted as their own control) and the short duration of the washout period between treatments (1 day). The short washout period was required for feasibility reasons, but we cannot exclude a potential residual effect of one intervention on the other. One positive was that the study was performed in a controlled environment, with patients hospitalized for the duration of the study, allowing consistent and reliable measurements under physiotherapist supervision.
Conclusions
This first clinical study documented the risks and benefits of short-term use of the new airway clearance device Simeoxin patients with obstructive lung diseases. The addition of the device to manual CP may have the potential to facilitate increased mucus clearance from the first drainage session, but this needs to be investigated in larger, well-controlled clinical trials.
Acknowledgements
Medical writing assistance was provided by Nicola Ryan, independent medical writer, funded by PhysioAssist.
List of abbreviations
ACD: airway clearance device; ACT: airway clearance technique; AE: adverse event; CF: cystic fibrosis; COPD: chronic obstructive lung disease; CP: chest physiotherapy; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; ITT: intention-to-treat; PaCO2: arterial carbon dioxide pressure; PaO2: arterial oxygen pressure; SD: standard deviation; SpO2: oxygen saturation
Ethics approval and consent to participate
All study participants provided written consent. The study protocol was approved by the CPP Sud-Méditerranée I ethics committee in Marseille, France (Ref: 2014-A00079-38).
Conflicts of interests
Laurent Morin is an employee of Physio-Assist. Philippe Giovannetti and Martine Reynaud-Gaubert have no conflict of interest to declare.
Funding
There was no funding body in the design of the study and data collection. Data management, statistical analysis and preparation of the clinical study report were performed by a CRO, funded by Physio-Assist.
Authors contributions
MRG designed the research and supervised the study. PG performed the study and data collection. All authors made contributions in interpretation of results, editing and revision of the manuscript. All authors read and approved the final manuscript.
References
1. Castellani C, Duff AJA, Bell SC, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17(2):153-78.
2. McIlwaine M, Bradley J, Elborn JS, et al. Personalising airway clearance in chronic lung disease. Eur Respir Rev. 2017;26(143):pii: 160086.
3. McIlwaine MP, Lee Son NM, Richmond ML. Physiotherapy and cystic fibrosis: what is the evidence base? CurrOpinPulm Med. 2014;20(6):613-7.
4. Flume PA, Robinson KA, O'Sullivan BP, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54(4):522-37.
5. Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database Syst Rev. 2015(12):Cd001401.
6. Giles DR, Wagener JS, Accurso FJ, et al. Short-term effects of postural drainage with clapping vs autogenic drainage on oxygen saturation and sputum recovery in patients with cystic fibrosis. Chest. 1995;108(4):952-4.
7. Main E, Prasad A, Schans C. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database Syst Rev. 2005(1):Cd002011.
8. McCormack P, Burnham P, Southern KW. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst Rev. 2017;10:Cd009595.
9. McIlwaine M, Wong LT, Chilvers M, et al. Long-term comparative trial of two different physiotherapy techniques; postural drainage with percussion and autogenic drainage, in the treatment of cystic fibrosis. PediatrPulmonol. 2010;45(11):1064-9.
10. Miller S, Hall DO, Clayton CB, et al. Chest physiotherapy in cystic fibrosis: a comparative study of autogenic drainage and the active cycle of breathing techniques with postural drainage. Thorax. 1995;50(2):165-9.
11. Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. 2015(11):Cd008351.
12. Lee AL, Burge AT, Holland AE. Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. 2017;9:Cd011699.
13. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):pii: 1700629.
14. Myers LB, Horn SA. Adherence to chest physiotherapy in adults with cystic fibrosis. J Health Psychol. 2006;11(6):915-26.
15. Abbott J, Dodd M, Bilton D, Webb AK. Treatment compliance in adults with cystic fibrosis. Thorax. 1994;49(2):115-20.
16. Morrison L, Milroy S. Oscillating devices for airway clearance in people with cystic fibrosis. PaediatrRespir Rev. 2018;25:30-2.
17. Radtke T, Boni L, Bohnacker P, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. BMC Pulm Med. 2018;18(1):99.
18. Sokol G, Vilozni D, Hakimi R, et al. The Short-Term Effect of Breathing Tasks Via an Incentive Spirometer on Lung Function Compared With Autogenic Drainage in Subjects With Cystic Fibrosis. Respir Care. 2015;60(12):1819-25.
19. Wallaert E, Perez T, Prevotat A, et al. The immediate effects of a single autogenic drainage session on ventilatory mechanics in adult subjects with cystic fibrosis. PLoS One. 2018;13(3):e0195154.
20. Bernard E, Israel L, Debris MM, et al. [Mucoviscidosis and idiopathic spontaneous pneumothorax]. J Fr Med ChirThorac. 1962;16:105-9.
21. Flume PA, Strange C, Ye X, et al. Pneumothorax in cystic fibrosis. Chest. 2005;128(2):720-8.
22. McIlwaine M, Button B, Dwan K. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database Syst Rev. 2015(6):Cd003147.
23. Bradley JM, Moran FM, Elborn JS. Evidence for physical therapies (airway clearance and physical training) in cystic fibrosis: an overview of five Cochrane systematic reviews. Respir Med. 2006;100(2):191-201.
24. Volsko TA. Airway clearance therapy: finding the evidence. Respir Care. 2013;58(10):1669-78.
25. Oermann CM, Swank PR, Sockrider MM. Validation of an instrument measuring patient satisfaction with chest physiotherapy techniques in cystic fibrosis. Chest. 2000;118(1):92-7.
Received: : January 21, 2020;
Accepted: Feb 11, 2020;
Published: Feb 13, 2020.
To cite this article : Giovannetti.P, Morin L, Gaubert MR.Safety and Efficacy of an Innovative Airway Clearance Device Versus Manual Chest Physiotherapy Techniques For Airway Secretion Clearance: a Feasibility Study. European Journal of Respiratory Medicine. 2020: 1:2.
©Giovannetti.P et al. 2020.
