Research Article/ Open Access
DOI: 2021; 3(1): 172 -177. doi: 10.31488/EJRM.113
The Effect of Nasal High Flow Therapy on Minute Ventilation in Chronic Obstructive Pulmonary Disease
Sowho MO*, Galiatsatos P, Guzman M, Hansel NN, Jun JC, Neptune ER, Biselli P, Kirkness JP
1. Johns Hopkins Sleep Disorders Center, Division of Pulmonary, Critical Care and Sleep Medicine, Baltimore, Maryland 21224, USA
*Corresponding author: Mudiaga Sowho MD MPH, Johns Hopkins School of Medicine, Johns Hopkins Sleep Disorders Center, 5501 Bayview Circle, Baltimore, MD, USA, Tel: +1 (401) 550-6264
Abstract
Introduction: Nasal high-flow therapy (HFT) has been shown to improve daytime breathing mechanics in healthy adults as well as the lung function and quality of life in chronic obstructive pulmonary disease (COPD) patients. Method: We hypothesized that improved breathing mechanics with HFT may further reduce minute ventilation (i.e. decreased work of breathing) during sleep in patients with COPD. In COPD participants we examined the dose effect of HFT (within night randomization of HFT level; 0, 10, 20 and 30L/min) on minute ventilation, oxyhemaglobin saturation and transcutaneous carbon dioxide during wake and sleep. We assessed overnight polysomnography with and without HFT on two separate nights. Paired t-tests were used to compare overnight sleep quality with and without HFT. The association between ventilatory variables and HFT level was assessed using regression analysis. Results: During sleep, HFT decreased minute ventilation by 0.63±0.02L/min per 10L/min nasal airflow by reducing tidal volume (37±6mL per 10L/min; p<0.001) without affecting respiratory rate (p=0.9) or arterial CO2 (p=0.7). In contrast, during wakefulness reductions in minute ventilation (0.85±0.04L/min per 10L/min) was due to respiratory rate reduction along with prolongation in expiratory time. Conclusion: The reduction in minute ventilation is greater with higher dead-space volumes (r=0.50; p<0.02) and during wakefulness suggesting that ventilatory responses to HFT are mediated through a reduction in dead-space ventilation. The reduction in ventilation in response to HFT is large enough to reduce respiratory loads. Reducing respiratory loads may avert muscle fatigue, preserve respiratory function, or prevent development of respiratory failure.
Introduction
In COPD patients, disease progression leads to the development of hypoventilation during sleep, leading to hypercapnia, thus increasing their morbidity and mortality [1,2]. To reduce mortality and reduce morbidity associated with hypercapnia treatment strategies have been devised to support ventilation [3-8]. Non-invasive ventilation (NIV) via a nasal or face mask remains the most effective method to improve nocturnal hypoventilation [9-11]. However, simpler or more manageable methods for preventing nocturnal hypoventilation during sleep are needed to improve health outcomes in patients with COPD.
We have recently shown that high flow therapy (HFT), warmed humidified air through a nasal cannula, can markedly improve breathing mechanics during sleep in normal individuals and children and adults with mild to severe upper airway obstruction[12,13]. Moreover, in hypercapnic COPD patients, application of HFT during the daytime wakefulness was associated with decreased pCO2, reductions in respiratory rate and work of breathing[14,15], thus indicating that HFT may reduce minute ventilation. However, there are no studies showing how ventilation changes in response to HFT in patients with COPD.
In this study we determined the effects of HFT on minute ventilation and transcutaneous CO2 levels over short periods during sleep and wakefulness in patients with COPD. We hypothesized that HFT would reduce ventilation during sleep and that there is a dose dependent effect of HFT on ventilation. Furthermore we determined the feasibility for the use of HFT during sleep in the laboratory and at home.
Methods
Subjects
Twenty adults with mild to severe COPD according to GOLD criteria [2,16] gave informed consent to participate in a study consisting of up to three non-randomized overnight sleep studies at the Johns Hopkins Sleep Disorders Center. Fifteen of the 20 participants completed the overnight protocols and were included in the analysis. Johns Hopkins Medicine Institutional Review Board (IRB Committee 5) approved the protocol. Exclusion criteria were presence of obstructive sleep apnea (>10 apneas-hypopneas/hr), an oxyhemoglobin saturation of <90% during the day or use of supplemental oxygen or decompensated cardiovascular or metabolic disease.
Materials and procedures
Polysomnography and ventilatory measurement
Overnight assessments were conducted by a full-montage in-laboratory polysomnographic recording including EEG, EOG, submental-EMG, ECG and transcutaneous CO2 with surface electrodes. Airflow signals were collected using a light-weight low resistance pneumotachograph connected to a nasal or full face mask [17]. In total, three overnight studies were conducted, one with and one without the HFT and one with intermittent HFT.
Nasal high flow therapy (HFT)
A custom nasal airflow system (ResMed, Bella Vista, Australia) was used to deliver flow levels between 10 and 30L/minute at the nose through a cannula (Figure 1). A built-in heating pad and humidifying chamber as well as a heating coil in the tubing regulated the temperature and humidity of the air delivered via the cannula outlet to a temperature of 30-35°C and relative humidity of 85-90%.
Usability of Nasal High Flow Therapy at Home: A subgroup of 10 individuals underwent a 20-30 minute instructional session on the usage of HFT. Each participant took a HFT device fitted with a data logger home for one week. The data logger was used to record total time on HFT device (TToD) and effective usage (EFF). Effective usage was defined as TToD minus any nasal cannula dislodgement time. Device usage diaries and Likert scale acceptability questionnaires were used to determine subjective usage and HFT acceptability.
Protocol
The initial visit included a history and physical examination, lung function testing followed by the polysomnographic study. At the second visit the participants additionally wore the HFT nasal interface and full-face mask. During wakefulness, HFT was delivered for 10 minute periods (10, 20, and 30L/min) in a random order each bracketed by 10 minutes off HFT (0L/min; Figure 2). At the conclusion of the wakefulness measurements the lights were turned off and the subject allowed to fall asleep. Following the onset of stable sleep we repeated the series of measures for the application of the HFT. In a subgroup of 6 individuals, a third overnight PSG study was conducted with the HFT in lab overnight. During entire overnight study with HFT, airflow was monitored with a nasal cannula rather than the full face mask.
Analysis
Polysomnography
All studies were analyzed for sleep stage, arousals and respiratory related events according to the most recent published American Academy of Sleep Medicine scoring criteria [18]. Greater than 15 seconds of EEG theta activity was considered sleep onset for the purpose of excluding confounds of sleep from ventilation measurement during wakefulness.
Minute ventilation measurement
Minute ventilation (VE) was calculated from breath-by-breath measurements of tidal volume (VT) and respiratory rate (RR) from 2-5 minute periods of steady state breathing at each HFT level (0, 10, 20 and 30L/min) in both wake and sleep states. Additionally, maximum inspiratory airflow (VImax), inspiratory and expiratory time (TI and TE), and inspiratory duty cycle (TI/TTOT) were extracted from the same breath-by-breath data [19]. Anatomical dead-space was determined using the Fowler equation (VD=-169+[218*height(m)]) [20]. Sleep variables (including total sleep time [TST], sleep stages, sleep efficiency, arousal and respiratory event indices) for the entire night on HFT were compared to the baseline night without HFT.
Statistics
Analysis of variance (ANOVA) was used to examine the effect of HFT level on ventilation, tidal volume, respiratory rate and TcCO2 during wake and sleep. Secondary, each breath analyzed was coded for sleep state, HFT level, and a subject identifier. General linear modeling (mixed-effects regression analysis) was applied to determine the independent effect of the level of HFT on respiratory airflow (VImax) and timing (TI, TE, TI/TTOT, RR) during wake and sleep. Subject identifier was included as a random factor to account for resting level of ventilation. Simple linear regression analysis was used to determine the association of VD with VE and the association of V¬E with HFT. Paired student t-tests were used to compare measures of sleep quality with and without HFT. A level of α<0.05 was considered significant.
Results
Only fifteen of the twenty subjects had adequate data to be included in the group analysis. In the five excluded subjects we did not obtain adequate ventilatory measurements for each level of HFT during sleep, these subjects were similar with regard to age, BMI or severity of COPD. Additionally, there were eight subjects who were unable to maintain wakefulness at the outset of the protocol with HFT (<40 mins). The subjects whom initiated sleep during the wakefulness recording did not differ from those in whom all data were obtained with regard to age, BMI or severity of COPD.
Demographic, anthropometric, polysomnographic and pulmonary function statistics for all fifteen COPD participants (5 males and 10 females) are displayed in Table 1. Mean age was 55.4±9.1 years and mean BMI was 28.5±8.4 kg/m2. Lung function was characterized by a mean FEV1 of 1.7±0.6L and FEV1/FVC of 61±16.4%. Similarly, lung volumes were elevated in the subjects, with an average total lung capacity (TLC) of 5.2±1.1L. A total of 6102 breaths from wake and sleep were analyzed for the entire group, averaging 435±32 breaths per subject. During the sleep periods, on average there were 241±20 breaths per subject analyzed compared to 209±14 breaths per subject during the wake periods.
Effect of HFT on minute ventilation during sleep
In 14 of the 15 individuals there was a significant dose dependent decrease in minute ventilation with increasing HFT levels during sleep, with an individual dose response range from -0.25 to -1.10 L/min per 10L/min HFT. For the group as a whole, average minute ventilation decreased by 0.63±0.02 L/min per 10 L/min HFT (p<0.001; Figure 3) and average tidal volume decreased by 37±1 mL per 10 L/min HFT (p<0.001; Figure 4). HFT did not change respiratory rate, inspiratory time or inspiratory duty cycle. Compared to baseline breathing (HFT=0 L/min), there was no change in TcCO2 at HFT flow rates of 10, 20 or 30 L/min (Figure 5). The reduction in minute ventilation was greater in individuals with higher dead-space volume. As can be seen in Figure 6, patients with a higher dead-space volume had a higher minute ventilation during NREM sleep (r2=0.32; p<0.03) and the reduction in ventilation with HFT trend towards greater values in those individuals with increased VD (r2=0.24; p=0.07).
Table 1.Anthropometrics, Baseline Polysomnography and Lung Function.
| Anthropometrics | |
| Age (yr) | 55.4.± 9.1 |
| Sex | 5 M / 10 F |
| Height (cm) | 176.6 ± 11.4 |
| Weight (kg) | 78.4 ± 15.6 |
| BMI (kg/m2) | 28.5 ± 8.4 |
| Polysomnography | |
| AHI (events/hr) * | 10.3 ± 7.4 |
| AI (events/hr) | 0.5±2.0 |
| HI (events/hr) | 8.9±8.7 |
| SaO2 (%) | 93±3 |
| Min SaO2 (%) | 89±3 |
| Lung Function | |
| FEV1/FVC(%) * | 61 ± 16.4 |
| FEV1 (L) | 1.7 ± 0.6 |
| TLC (L) | 5.2 ± 1.1 |
BMI=Body mass index; AHI=Apnea Hypopnea Index; AI=Apnea Index; HI=Hypopnea index; SaO2=baseline oxyhemoglobin saturation; Min SaO2=overnight minimum SaO2.
Effect of HFT during wakefulness
In the subgroup of seven subjects for whom sufficient wakefulness data were obtained, all subjects had a linear dose dependent decrease in ventilation with increasing HFT (individual range: 0.36 to 1.46 L/min per 10L/min HFT). Group mean minute ventilation without HFT was 6.9±0.7 L/min and decreases by a mean of 0.85±0.04 L/min per 10 L/min HFT (p<0.001). There was a decrease in TcCO2 of -1.3±0.1 mmHg (p=0.02) at 10 L/min HFT which was maintained at 20 and 30 L/min. There was a linear decrease per 10 L/min HFT in TI/TTOT (-0.02±0.00 a.u.; p<0.001) and an increase in TI (0.03±0.01 s; p<0.001), TE (0.25±0.02 s; p<0.001) and TTOT (0.27±0.02 s; p<0.001).
Effect of HFT on sleep quality and usability
PSG sleep characteristics in those that used HFT for the entire night had similar demographic and anthropometric characteristics compared to those in the entire study group. Use of HFT produced no change in any measures of sleep quality including TST, sleep efficiency, sleep or REM onset latency, wake after sleep onset, sleep stages, arousal and respiratory event indices, and baseline or minimum SaO2 (Table 2).
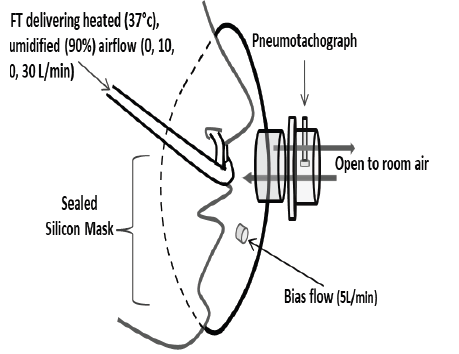
Figure 1.Measurement of minute ventilation with nasal high flow therapy (HFT). A custom nasal cannula and full-face mask attached to a light weight low dead-space, low resistance pneumotachograph was used to measure respiratory airflow at varying levels of HFT. A small bias-flow through the mask prevented accumulation of expired gases in the mask.
Table 2.Overnight Sleep Quality with High Flow Therapy.
| Baseline | HFT (@20L/min) | |
|---|---|---|
| Polysomnography | ||
| TST (min) | 395 ± 18 | 378 ± 32 |
| SE (%TST) | 84 ± 3 | 80 ± 6 |
| Sleep onset latency (min) | 8 ± 4 | 11 ± 5 |
| REM onset latency (min) | 91 ± 11 | 88 ± 16 |
| WASO (min) | 67 ± 15 | 80 ± 30 |
| Sleep Stages | ||
| N1 (%) | 11 ± 3 | 13 ± 3 |
| N2 (%) | 50 ± 6 | 45 ± 4 |
| N3 (%) | 15 ± 5 | 29 ± 9 |
| REM (%) | 24 ± 4 | 22 ± 3 |
| AHI (events/hr) | 8.3 ± 3.1 | 8.4 ± 4.3 |
| ATDBT (%) | 15 ± 5 | 15 ± 7 |
| Arousal Index (events/hr) | 5.1 ± 1.8 | 4.2 ± 1.6 |
| SaO2 (%) | 95 ± 1 | 95 ± 1 |
| Min SaO2 (%) | 87 ± 2 | 86 ± 1 |
TST=total sleep time; SE=Sleep efficiency; WASO= wake after sleep onset; AHI=Apnea Hypopnea Index; ATDBT=Apnea time to disordered breathing time; SaO2=baseline oxyhemoglobin saturation; Min SaO2=overnight minimum SaO2.
Additionally, ten individuals used HFT in the home for a one week period with mean daily. HFT usage (both subjective reported and objectively recorded) for each individual reported in Table 3. Eight of the ten individuals used the HFT >4hr per night and the group mean nightly time on HFT was 6.0±0.9 hr/night (range 1.1-8.8 hr/night). After subtracting periods of cannula dislodgement, five of the ten subjects were found to have effective usage time >4hrs/night (range 0.2-5.9 hr/night). The subjectively reported usage time on average was 1.3±0.4 hr/night greater than the objectively recorded time. Nine of the ten participants rated usage of the HFT as very acceptable with an average assessment rating of 8 on the 10 point Likert scale.
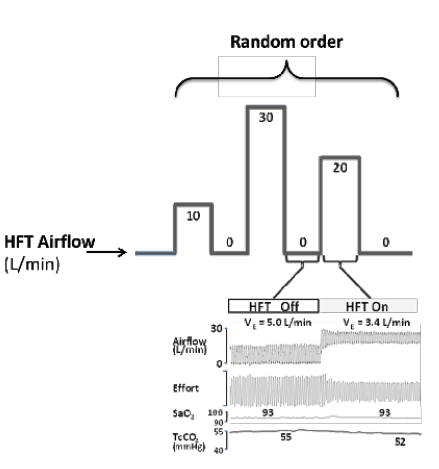
Figure 2.Schematic illustration of the protocol. Initially, during wakefulness, high flow therapy (HFT) was applied via the nasal cannula at either 10, 20 or 30 L/min randomized for each subject and returned to 0 L/min between each level. During sleep the same order of HFT was repeated. A raw data recording example (lower inset) shows a decrease in airflow, respiratory effort and TcCO2 at 20 L/min HFT.
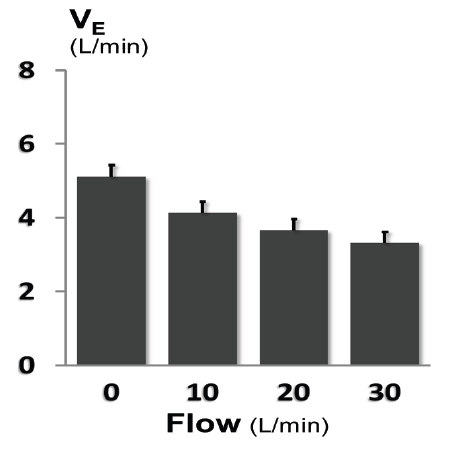
Figure 3.Dose dependent decrease in ventilation with high flow therapy. During sleep there was a progressive decrease in resting ventilation with increasing HFT level (p<0.001). At each level of HFT the ventilation was lower during sleep compared to wake.

Figure 4.Effect of high flow therapy on tidal volume and respiratory rate. A) Tidal volume decreased in a dose dependent fashion with increasing HFT levels during sleep (p<0.001). B. Respiratory rate did not change with HFT during sleep (p=0.9).
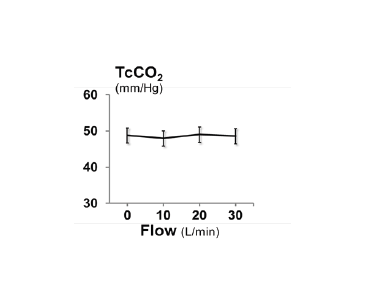
Figure 5.The effect of high flow therapy on transcutaneous carbon dioxide. For the group as a whole there was no change in TcCO2 with HFT during sleep (p=0.7). Values are mean ± SE.
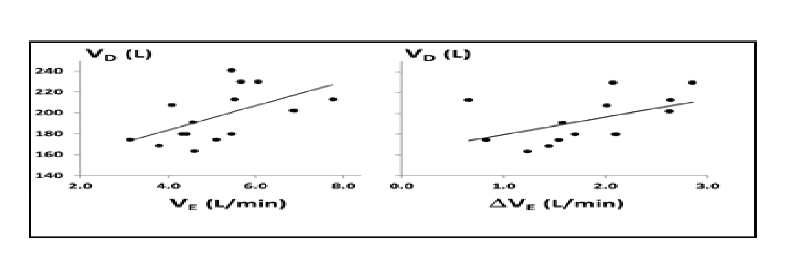
Figure 6.The Anatomical Dead space and Ventilation with and without HFT. There was significant association between anatomical dead space and resting minute ventilation during NREM sleep (left panel; p<0.03). There was a trend towards an association between the anatomical dead space and the change in minute ventilation with 30L/min HFT (right panel; p=0.07).
Table 3.High Flow Therapy Adherence and Usability.
| Objective | Subjective | |||
|---|---|---|---|---|
| ID | TToD (hrs) | EFF (hrs) | RPU (hrs | Acceptability (Likert scale) |
| 1 | 5.1 | 4.4 | 6.0 | 9 |
| 2 | 8.8 | 5.5 | 7.9 | 9 |
| 3 | 7.3 | 5.4 | 7.4 | 9 |
| 4 | 3.1 | 2.5 | 4.9 | 9 |
| 5 | 7.7 | 5.1 | 9.9 | 8 |
| 6 | 8.4 | 4.0 | 10.7 | 9 |
| 7 | 6.0 | 3.5 | 8.0 | 10 |
| 8 | 4.3 | 3.2 | 6.5 | 9 |
| 9 | 1.1 | 0.2 | 2.0 | 1 |
| 10 | 8.2 | 5.9 | 9.8 | 9 |
TToD=total time on device (average hrs per night); EFF=Effect time on device (average hr on device adjusted for cannula dislodgement); RPU= self-reported usage time (average hrs/night); Acceptibility=A 10 point Likert scale (1=not acceptable, 10=highly acceptable).
Discussion
The main finding in this study was that in COPD patients, HFT reduced minute ventilation in a dose dependent fashion by ~10 to 40% at airflow rates of 10-30L/min during sleep without an increase in TcCO2. The reduction in minute ventilation is greater in individuals with higher dead-space a volume, indicating that HFT’s mechanisms of action is partly mediated through a reduction in dead-space ventilation. The use of HFT during sleep did not change sleep time or lead to altered sleep architecture, demonstrating no significant sleep disruption. The majority of patients found that HFT is acceptable for use during sleep.
Ventilatory responses to HFT during sleep and wakefulness
We found that HFT at 30L/min decreased minute ventilation in our COPD individuals more than expected (30-40%) based on previous studies [21] in normal individuals in whom we observed only a 15-20% reduction in ventilation during sleep at similar flow rates. There are several possible explanations for the greater reduction in ventilation seen in patients with COPD. First, HFT may have washed out dead-space of the airway. Since COPD patients often have a greater proportion of dead-space ventilation [22] the effect of reducing dead-space ventilation may be greater than in those with lower dead-space ventilation. This possibility is supported by our finding that individuals with greater anatomical dead-space had a larger reduction in minute ventilation with HFT. Second, it is similarly possible that individuals with loaded respiratory systems exhibit greater reductions in ventilation than those individuals with normal loads [23]. Although we did not quantify respiratory loads in our patient population, our subset analysis comparing HFT responses between wakefulness and sleep supports the possibility that increased respiratory loads at baseline may influence HFT responses. During wakefulness, minute ventilation was ~20% greater compared to sleep indicating a greater respiratory load at wakefulness. There was however no difference in ventilation between wake and sleep at the 30L/min HFT condition because patients decreased minute ventilation in response to HFT to greater degrees during wakefulness than during sleep. Taken together, it appears that ventilatory responses to HFT are more impressive in COPD compared to normal healthy individuals [24] and that either increased respiratory loads or increased dead-space ventilation may play a role in these responses.
HFT differs from both CPAP and NIV, yet resembles tracheal insufflation
Several studies have examined the effect of continuous or bi-level positive airway pressure support (CPAP or BiPAP) on ventilation and gas exchanges in patients with COPD. O’Donoghue et al. demonstrated that increasing levels of CPAP actually increased minute ventilation without a change in arterial blood gases [25], indicating that a patients’ ventilatory response to CPAP would differ from their response to HFT. Likewise, HFT would also differ from responses to BiPAP. During BiPAP, dead-space volume is increased since nasal or facial masks impose added dead-space volume. Improvements in alveolar ventilation must first overcome this added dead-space volume, which often requires the application of either high tidal volumes or high trans-pulmonary pressures, neither of which is generally well tolerated. The findings of our study show that minimal reduction in arterial CO2 can be achieved by lowering dead-space volume with nasal HFT. A reduction in dead-space volume to assist breathing has been used in patients with tracheotomies by insufflating fresh air into the tracheal tube. In these studies, reductions in dead-space volumes as low as 40 ml have been shown either to decrease arterial CO2 from 46 to 40 mmHg, if tidal volume remained unchanged, or to reduce minute ventilation and work of breathing with no or only minimal reductions in arterial CO2 [26]. The ventilatory responses to nasal HFT, therefore, resemble more those of tracheal gas insufflation. Thus, the principles of tracheal gas insufflation appear more suitable to explain physiologic and clinical responses of nasal HFT than CPAP and NIV.
Measurement of minute ventilation in COPD during sleep and wake
Accurate measurements of airflow and ventilation during sleep in patients with respiratory disorders require the use of sealed full face masks and pneumotachographs. These methods often increase airway dead-space and/or resistance, altering or restricting long term assessment of minute ventilation during sleep [27]. We have employed a unique light weight pneumotachographic sensor which minimizes the dead-space and resistance for airflow measurement [17]. In addition the mask volume was flushed with a small yet sufficient bias flow. It is unlikely that the mask contributed to any measurement bias as in the current study, ventilation during sleep was remarkably low compared to previously reported levels for COPD patients with moderate to severe airflow obstruction or with hypercapnia [28,29]. The reduced minute ventilation during sleep was primarily due to low tidal volumes (~300 ml) and not reductions in respiratory rate, reflecting a shallower breathing pattern during NREM sleep compared to wakefulness.
Effect of HFT on overnight sleep quality and usability
Usage of HFT was assessed in the laboratory under monitored and controlled environmental conditions as well as in the home setting where usage conditions may vary widely[30]. In the laboratory, there were no night to night differences in the sleep architecture demonstrating that the high flow and the nasal cannula do not acutely disrupt sleep. In the home setting, most people initially intended to use the nasal cannula during sleep. One individual reported difficulty using the HFT due to a concurrent episode of rhinorrhea. There was no evidence to suggest that the runny nose was associated with the use of the HFT as this individual’s symptoms did not worsen with HFT usage. While it was found that HFT was very well accepted, our data suggest that nasal cannula dislodgement time over estimates the time of effective therapy. We did not titrate the HFT to an individually optimized flow rate in the current protocol since most individuals found the level used very acceptable. It is expected, however, that individually adjusting the HFT over time to optimize comfort would improve adherence.
Strengths and limitations
There are several strengths in the current study: The first strength is our study design consisting of randomized sequence of each experimental condition. The second is the use of polysomnography during the extended wakefulness period allowing us to recognize that more than half of our individuals dozed off during the wakefulness trial. Third, we quantified minute ventilation using a low resistance and low dead-space pneumotachograph attached to a sealed custom made full face mask allowing us to do a breath by breath analysis during transitional and steady state periods. The set up did not create any discomfort nor did it interfere with our ability to execute the protocol, which is best demonstrated by the ease with which subjects fell asleep.
We acknowledge several limitations. First, most of our patients had only a mild degree of nocturnal hypoventilation and only two patients were severely airflow limited (FEV1<1L; <40% of predicted) with evidence of hypercapnea at rest (TcCO2>50mmHg). Both of these individuals exhibited the average response to HFT, suggesting that therapy may be equally effective across a spectrum of COPD severity. Second, we did not directly measure arterial blood gases. However, transcutaneous CO2 monitoring can accurately detect changes in arterial pressure[31,32]. Third, the HFT trials lasted 5-10 minutes, which was sufficient to achieve steady state responses for ventilation but the periods were too short to reliably determine the overall effects of HFT on sleep characteristics. Fourth, we did not have an age and weight matched control group without COPD. Therefore it remains unclear whether greater responses to HFT as compared to previous reports in young individuals with normal lungs are in fact specific to COPD or partly due to age related changes in breathing mechanics or both.
Clinical implications
There are three major clinical implications of our findings. First, HFT is an easy to implement method to reduce ventilation without altering arterial blood gases. Thus, it may be a viable alternative to non-invasive ventilation for unloading breathing, both in the acute setting and in long-term use settings. Second, increased breathing efficiency by decreasing the proportion of dead-space ventilation with HFT could be used to prevent development of respiratory failure. Third, a 30-40% reduction in minute ventilation with HFT during sleep may reduce the energy cost of breathing and may help prevent or limit cachexia as lung function declines with disease progression.
Acknowledgements
The authors would like to acknowledge Drs. A. R. Schwartz and P. L. Smith and other staff of Johns Hopkins Sleep Laboratory for logistic support and sleep laboratory operations.
Support
Support for this study and the flow generators used to deliver HFT were provided by ResMed Science Center. Support for this study was also provided by AHA12SDG8100000, NHLBI–HL077137.
References
1. Van Remoortel H, M Hornikx, D Langer, et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:30-38.
2. Vestbo J, SS Hurd, AG Agusti, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347-365.
3. Porszasz J, R Cao, R Morishige, et al. Physiologic Effects of an Ambulatory Ventilation System in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2013;188:334-342.
4. Chatila W, T Nugent, G Vance, et al. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004;126:1108-1115.
5. Lopes AJ, FP Nery, FC Sousa, et al. CPAP decreases lung hyperinflation in patients with stable COPD. Respir Care. 2011;56:1164-1169.
6. Barakat S, G Michele, P Nesme, et al. Effect of a noninvasive ventilatory support during exercise of a program in pulmonary rehabilitation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:585-591.
7. Corner E, R Garrod. Does the addition of non-invasive ventilation during pulmonary rehabilitation in patients with chronic obstructive pulmonary disease augment patient outcome in exercise tolerance? A literature
8. Garrod R, C Mikelsons, EA Paul, et al. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. Am J Respir Crit
9. Lightowler JV, JA Wedzicha, MW Elliott, et al. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic r
10. Peter JV, JL Moran, J Phillips-Hughes, et al. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367:1
11. Peter JV, JL Moran, J Phillips-Hughes, et al. Noninvasive ventilation in acute respiratory failure--a meta-analysis update. Crit Care Med. 2002;30:555-562.
12. McGinley B, A Halbower, AR Schwartz, et al. Effect of a high-flow open nasal cannula system on obstructive sleep apnea in children. Pediatrics. 2009;124:179-188.
13. McGinley BM, SP Patil, JP Kirkness, et al. A nasal cannula can be used to treat obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:194-200.
14. Nilius G, KJ Franke, U Domanski, et al. Effects of Nasal Insufflation on Arterial Gas Exchange and Breathing Pattern in Patients with Chronic Obstructive Pulmonary Disease and Hypercapnic Respiratory Failure.
15. Nilius G, T Wessendorf, J Maurer, et al. Predictors for Treating Obstructive Sleep Apnea with an Open Nasal Cannula System. Chest. 2009;137:521-528.
16. Lange P, JL Marott, J Vestbo, et al. Prediction of the Clinical Course of Chronic Obstructive Pulmonary Disease, Using the New GOLD Classification: A Study of the General Population. Am J Respir Crit Care Med
17. Kirkness JP, M Verma, BM McGinley, et al. Pitot-tube flowmeter for quantification of airflow during sleep. Physiol Meas. 2011;32:223-237.
18. Berry RB, R Brooks, CE Gamaldo, et al. 2012. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, Version 2.0, Version 2.0 ed. American Academy of Sl
19. Wei T, MA Erlacher, P Grossman, et al. Approach for streamlining measurement of complex physiological phenotypes of upper airway collapsibility. Comput Biol Med. 2013;43:600-606.
20. Kars AH, JM Bogaard, T Stijnen, et al. Dead space and slope indices from the expiratory carbon dioxide tension-volume curve. Eur Respir J. 1997;10:1829-1836.
21. Calverley PMA. Impact of sleep on respiration. Eur Resp Monograph. 1198;10:9-27.
22. Rodriguez-Roisin R, M Drakulovic, DA Rodriguez, et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol. 2009;106:1902-1908.
23. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709-721.
24. Mundel T, S Feng, S Tatkov, et al. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol. 2013;114:1058-1065.
25. O'Donoghue FJ, PG Catcheside, AS Jordan, et al. Effect of CPAP on intrinsic PEEP, inspiratory effort, and lung volume in severe stable COPD. Thorax. 2002;57:533-539.
26. Tagaito Y, H Schneider, CP O'Donnell, et al. Ventilating with tracheal gas insufflation and periodic tracheal occlusion during sleep and wakefulness. Chest. 2002;122:1742-1750.
27. Pillar G, A Malhotra, R Fogel, et al. Airway mechanics and ventilation in response to resistive loading during sleep: Influence of gender. Am J Respir Crit Care Med. 2000;162:1627-1632.
28. O'Donoghue FJ, PG Catcheside, DJ Eckert, et al. Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol. 2004;559:663-673.
29. O'Donoghue FJ, PG Catcheside, EE Ellis, et al. Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J. 2003;21:977-984.
30. Sowho MO, MJ Woods, P Biselli, et al. Nasal insufflation treatment adherence in obstructive sleep apnea. Sleep Breath. 2014 [ePub ahead of print].
31. Storre JH, FS Magnet, M Dreher, et al. Transcutaneous monitoring as a replacement for arterial Pco(2) monitoring during nocturnal non-invasive ventilation. Respir Med. 2010.
32. Storre JH, B Steurer, HJ Kabitz, et al. Transcutaneous PCO2 monitoring during initiation of noninvasive ventilation. Chest. 2007;132:1810-1816.
Received: January 01, 2021;
Accepted: February 17, 2021;
Published: February 22, 2021.
To cite this article : Sowho M, Galiatsatos P, Guzman M, et al. The Effect of Nasal High Flow Therapy on Minute Ventilation in Chronic Obstructive Pulmonary Disease. European Journal of Respiratory Medicine. 2021; 3:1
© 2021 Mudiaga Sowho, et al.
