Protocol/ Open Access
DOI:10.31488/EJRM.139
The Role of High Frequency Chest Wall Oscillations Administered via the Vest® Airway Clearance System in Addition to Non-Invasive Respiratory Therapies in the Treatment of Patients with Acute Respiratory Failure and Hypersecretion: Monocentric, Parallel Group, Controlled Randomized Clinical Trial (VICOR)
Laura Cirilli MD4 , Nicoletta Golfi MD1 , Luca Guidelli MD1 , Teresa Renda MD1 , Debora Falcone N1 , Gabriella Tacconelli RT2 , Matilde Vespi RT2 , Stefania Arniani3 , Irene Guidelli1 , Martina Bonifazi MD4 , Raffaele Scala MD*1
1.Cardio-Neuro-Thoracic-Vascularc Department, Pulmonology and Respiratory Intensive Care Unit, Usl Toscana Sudest, San Donato Hospital, Arezzo, Italy
2.Physiotherapy, Usl Toscana Sudest, San Donato Hospital, Arezzo, Italy
3.Epidemiology and Statistics Usl Toscana Sudest, San Donato Hospital, Arezzo, Italy
4.Università Politecnica delle Marche, Ancona, Italy
*Corresponding author: Dr. Raffaele Scala, Principal Investigator, Cardio-Neuro-Thoracic-Vascularc Department, Pulmonology and Respiratory Intensive Care Unit, Usl Toscana Sudest, San Donato Hospital, Arezzo, Italy, Tel: +39 0575-255216; Fax: +39 0575-254545
Abstract
An excessive airway secretions burden is one of the major causes for NIRT failure in the setting of acute respiratory failure or acute on chronic respiratory failure. HFCWO is a system which enhances mucociliary clearance and could be effective in reducing the need for bronchoscopy and endotracheal intubation, mechanical ventilation duration, hospital and RICU stay duration and mortality rate. Here we present the protocol of our monocentric, parallel group, controlled randomized clinical trial aiming at evaluating if the application of HFCWO, as the assigned intervention, in a population of patients suffering from acute respiratory failure or acute on chronic respiratory failure and requiring NIRT could add some benefit in terms of health-related outcomes.
Keywords: HFCWO, NIV, HFNC, acute respiratory failure
Introduction
NIRT has demonstrated its efficacy in reducing endotracheal intubation and mortality in a wide spectrum of patients with ARF from different etiologies [1]. The presence of excessive airway secretions and the inability to autonomously discharge them could be a cause of NIRT failure, more proven for NIV than for HFNC [2,3]. The greatest evidence about benefits derived from NIV application regards COPD exacerbations with acidotic hypercapnic respiratory failure and the prevention of endotracheal intubation and invasive mechanical ventilation in this setting, acute pulmonary edema, immunodepressed patients and weaning from invasive mechanical ventilation [2]. Conversely, HFNC is favoured over NIV in patients with de novo hypoxemia even if the level of evidence is not strong. HFNC and NIV should not be considered alternative supportive tools; a combination of the two methods - HFNC and NIV- may be applied with success in an integrated and sequential fashion in different forms of ARF [3].
NIRT increases alveolar ventilation, reduces muscular work of breathing, respiratory rate and dyspnea intensity and improves oxygenation. It also reduces mortality and rate of endotracheal intubation. At the same time NIRT reduces adverse events related to invasive mechanical ventilation, such as ventilator associated pneumonia and shortens the length of hospital stay [4-7].
An excessive burden of airway secretions is associated with NIRT failure, especially in association with ineffective cough [8] which results in the need for invasive procedures during NIRT such as flexible bronchoscopy or endotracheal intubation [9]. Several studies have shown that a patient- tailored rehabilitative program is effective in reducing dyspnea, increasing health related quality of life, shortening the length of hospital stay and reducing healthcare expenditure; moreover, complete pulmonary rehabilitation programs provide psychosocial benefits in COPD patients. Respiratory physiokinesistherapy is deemed effective in patients affected from COPD and other chronic respiratory diseases [10-12].
High frequency chest wall oscillation (HFCWO) is a technology which aids airway secretions removal in patients with increased bronchial secretive burden and/or inability to spontaneously discharge secretions via the physiological mucociliary clearance system affected by different physiopathological factors.
The system consists of an air pump connected with an inflatable jacket that the patient wears around the chest. The pump generates and transmits oscillations to the jacket that compresses the thorax with a frequency variable from 5 to 25 Hz, facilitating mucus detachment from the bronchial walls and subsequently discharge of secretions from the airways with cough. The jacket must adhere to the thorax and be comfortable at the same time; the pressure range applied varies from 2 to 5 cm H2O [13-15]. One of the effects of HFCWO is generating airflow oscillation which are transmitted also in the intrapleural space as variations in intrapleural pressure which contributes to normal secretions discharge mechanisms. The use of this system does not require an active co- operation from the patient. Notwithstanding the great physiopathological rationale in improving mucociliary clearace, definite data from the literature are still lacking; nowadays strong evidence regarding the improvement of secretions’ discharge with acceptable tolerance profile is available only in selected cohorts such as in children with cystic fibrosis and in adults with amyotrophic lateral sclerosis [16-21]. Two studies demonstrated that oscillations produced by HFCWO reduce mucus viscosity and help its removal in patients affected by bronchiectasis and neuromuscular diseases [13,20].
To date, no clinical studies assessing safety and efficacy of HFCWO in the clinical setting of patients with ARF requiring NIRT are available in literature.
Information about the Medical Device Used in the Study
HFCWO will be delivered via The Vest® Airway Clearance System (HillRom, Batesville, Indiana, USA). This device consists in an air pump connected to an inflatable jacket that the patient wears around the thorax. Treatment sessions will last at least 15 minutes and will be administered to the patient three times in a day, setting a variable frequency of oscillations from 13 to 15 Hz according to the patient’s tolerance; pressure will be set from 2 to 5 cm H2O so that the jacket will be adherent to the thorax in a comfortable way [22].
Oscillations generated by HFCWO reduces mucus viscosity and facilitate its discharge from the airways. The device will be applied to critically ill respiratory patients by a physiotherapist with specialized expertise in the Pulmonologic setting also when provided with a RICU. During the treatment patient will maintain a seated position.
One experimental device The Vest® Airway Clearance System is intended to be used for the conduction of this study.
Study Endpoints
We hypothesize that HFCWO application could help spontaneous airway secretions removal in patients affected by ARF/acute on chronic respiratory failure, bronchial hypersecretion and who need NIRT. Study endpoints are derived from this hypothesis.
Primary Outcome
“Rate of patients undergoing bronchoscopy for the management of airway secretions during NIRT”: this end-point aims at evaluating if the addition of HFWCO to NIRT may reduce the need for bronchoscopy compared to the use of NIRT alone.
Secondary Outcomes
1. “Days of NIRT duration”: this end-point aims at evaluating if the addition of HFWCO to NIRT may reduce the length of non invasive respiratory supports compared to the use of NIRT alone;
2.“Days of RICU stay”: this end-point aims at evaluating if the addition of HFWCO to NIRT may reduce the length of RICU stay compared to the use of NIRT alone;
3.“Number of patients who meet criteria for endotracheal intubation and IMV”: this end- point aims at evaluating if the addition of HFWCO to NIRT may reduce the need for IMV compared to the use of NIRT alone;
4. “Number of patients who undergo endotracheal intubation for inability to self-managing secretions in patients without DNI indication and RICU mortality for DNI patients”: this end-point aims at evaluating if the addition of HFWCO to NIRT may reduce the need for IMV due to inability to self-managing secretions and RICU mortality compared to the use of NIRT alone;
5. “Change in Sputum volume”: this end-point aims at evaluating if the addition of HFWCO to NIRT may increase the amount of sputum compared to the use of NIRT alone.
6. “Comfort in using The Vest airway clearance system” in patients treated with HFCWO in addition to NIRT (Figure 1).
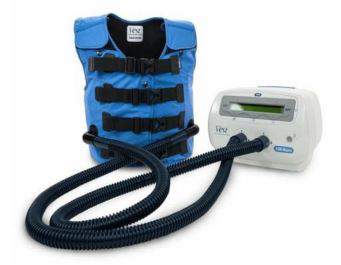
Figure 1:The Vest Airway Clearance System
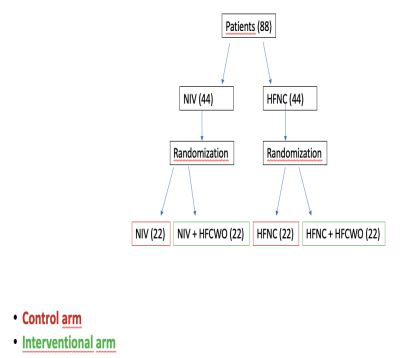
Figure 2:Study design
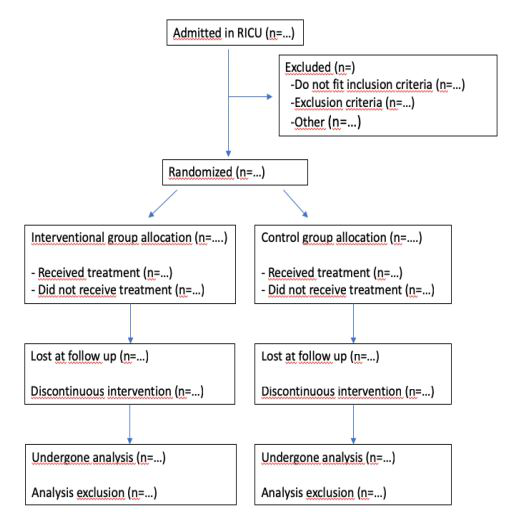
Figure 3:Patient flow diagram
7. “Post discharge PFT (FVC ml e % pred, FEV1 ml e % pred, DLCO % pred) - “PCEF”, “6 minute Walking Test” assessed 90 days after hospital discharge.
NIRT Failure Criteria
Bronchoscopy is required when the patient is unable to spontaneously remove secretions despite physiokinesistherapy (Cough score < 3), when gas exchange deterioration occurs during NIRT (defined as PaO2/FiO2<200 or PaO2 <60 and/or PaCO2 increase of 20%), and/or when there is radiological worsening such as development of lobar/multilobar/pulmonary atelectasis or worsening of pre-existing atelectasis.
Study Design and Duration
This is a monocentric parallel group controlled randomized clinical trial, ongoing in the RICU of “San Donato Hospital” in Arezzo (ITALY, Tuscany) involving patients admitted for ARF and bronchial hypersecretion. The expected study duration is estimated to be 18/24 months. This clinical trial is registered on www.clinicaltrial.gov identified with protocol-ID “VICOR2” and with clinicaltrial.gov-ID “NCT05751707”.
Randomization and Number of Patients to Enroll
This will be a randomized study.
To obtain an equal patient distribution between control and interventional arms a blind randomization sequence will be used. It will be available for consultation for the Principal Investigator and will be preserved in a sealed envelope locked in its office.
Since no data reference are available on the efficacy of HFCWO in reducing the rate of bronchoscopy in hypersecretive patients with ARF receiving NIRT, the sample size is calculated on the basis of the theoretical device’s capacity of reducing up to 50% the risk for NIRT failure because of incapacity to manage secretions. This risk is quantified as 80% in the study sample population.
We set the statistical power at 80% and type 1 error probability at 0.05: the planned sample size is 88 patients:
44 patients for control arm (n. 22 treated with NIV combined or not with HFNC; n. 22 treated only with HFNC)
44 patients for intervention arm [n. 22 treated with NIV combined or not with HFNC associated with the addition of HFCWO; n. 22 treated only with HFNC associated with the addition of HFCWO)]
Setting
The RICU included in the “Pulmonology and Respiratory Intensive Care Unit” of San Donato Hospital in Arezzo (ITALY, Tuscany) is equipped with 8 beds. RICU is defined as “a respiratory high specialized area of monitor and treatment of patients affected by acute respiratory failure by de novo respiratory cause and/or by acutely exacerbated chronic respiratory failure (Acute on Chronic Respiratory Failure), where commonly non invasive monitoring systems are used and where non invasive mechanical ventilation is preferentially, but not exclusively, used” [23]. Patients who need mechanical ventilation and monitoring are those affected by ARF due to pulmonary parenchymal diseases without co-existing multiorgan failure, with PaO2/FiO2 ratio < 300. RICU will also host patients with prolonged and/or difficult weaning from mechanical ventilation coming from conventional ICUs or patients already weaned and tracheotomized assessed for the chance of removing the tracheostomy cannula and still needing monitoring or invasive care. Also, RICU is the appropriate setting for patients with central hypoventilation syndromes or sleep apnea syndrome admitted for ARF or for patients with post-operative ARF. RICU works as a step-up unit for patients with mild to moderate ARF admitted in the general ward and as a step-down unit for those discharged from general ICU who need to prolong non invasive monitoring. This setting is provided with a nurse/patient ratio of 1:4. Every patient undergoes continuous vital sign monitor (heart rate, SpO2, respiratory rate, systemic arterial pressure); an arterial catheter is usually inserted to allow continuous monitor of invasive systemic arterial pressure and to facilitate ABG sampling. RICU allows the use of NIRT in patients with moderate respiratory acidosis but IMV only in tracheostomized patients. The physiokinesistherapy equipe has a central role in the multidisciplinary program of weaning from ventilation and adherence to NIRT. The medical team involved has expertise in RICU, respiratory physiopathology, mechanical ventilation and cardiorespiratory emergencies management.
Inclusion Criteria
• Age above 18 yo;
• Diagnosis of ARF or acute on chronic respiratory failure (including patients having home oxygen therapy, HFNC, NIV) both hypercapnic (PaCO2 > 45 mmHg; PaO2/FiO2<300) or hypoxaemic (PaCO2 <45 mmHg; PaO2/FiO2 <300);
• Informed consent from patient or legal tutor;
• Accessory respiratory muscles use;
• RR above 25 apm;
• Intermittent use of NIRT (NIV combined or not with HFNC or HFNC alone) since RICU admission (<20h per day)
• Kelly-Mattahy score ≤ 3 [24]
• Excessive bronchial secretions (clinical evaluation asking the patient to cough) and inability to efficiently remove secretions (evaluated with the PCEF measurement). A PCEF lower than 270 L/m is highly suggestive of inadequate cough which prevents the patient from adequately manage and remove airway secretions [21].
• Cough score < 3: in the case of inability to measure PCEF due to poor patient co-operation and/or weakness, the assessment of cough adequacy will be performed by a respiratory physiotherapist in a semiquantitative manner (the “Cough score” is based on the measurement of sputum volume produced after coughing three times. 1 point: less than 2 mL, 2 points: 2-6 mL, 3 points: more than 6 mL) [9,25].
Exclusion Criteria
• Patient unwillingness or incapability to provide informed consent;
• Need for continuous NIV (>20 h per day);
• Kelly-Matthay score > 3;
• Severe hemodynamic instability;
• Acute coronary syndrome;
• Psychomotor agitation unresponsive to analgo-sedation (RASS> 1);
• Contraindications to HFCWO use: pneumothorax (even when chest drainage is not required); severe chest wall deformities (pectus excavatum, pectus carinatum or pectus arcuatum); severe obesity (BMI>40 kg/m2); pregnancy; thoracic or abdominal surgery in the previous 6 weeks;
• Nasal swab positivity to Sars-CoV-2;
• Need for endotracheal intubation or urgent bronchoscopy for excessive airway secretions.
Treatment
NIRT arm (control arm): patients randomized into the control arm will be stratified according to the ventilatory support adopted, NIV combined or not with HFNC or HFCN alone, as described below:
• NIV combined or not with HFNC: in the case of NIV it will be delivered intermittently (up to a maximum of thrice a day) with sessions of varying duration according to clinical/blood gas response and patient tolerance with oronasal mask, total-face mask, nasal mask or helmet applying if necessary the interface rotational strategy to increase patient tolerance.
Ventilator setting: PSV or P-A/C mode according to clinician's judgment, using an ICU ventilator equipped with pressure and flow curve monitoring. Start applying a PS of 8cm H2O such that a Vte of 6-8 ml/Kg and a Respiratory Rate<25 acts/minute are achieved, use a FiO2 which allows to mantain SpO2 between 88 and 92%. The ventilator settings for each patient (PS, PEEP, inspiratory trigger, expiratory cycling) is adjusted based on the pressure and flow curves of the ventilator. The analysis of these curves is critical to optimize patient-ventilator interaction [26].
The patient can start to be weaned from NIV when he successfully passes the spontaneous breathing trial, fulfilling all the following vital parameters after 1 hour of spontaneous breathing with eventual oxygen supplementation, [27]:
-RR 8-24 apm
- Systemic systolic blood pressure >90 mmHg not sustained by vasopressors
- Body temperature between 36-37°C
- HR 50-90 bpm
-Kelly-Mattay score ≤ 2
-SpO2 between 88-92% with FiO2≤40%
- pH≥7.35
-Absence of severe dyspnea (Borg scale<4)
If all these criteria are achieved and the morning spontaneous breathing trial is successfully passed, the patient will be weaned from NIV. Weaning will begin first by discontinuing daily NIV sessions, then NIV usage will be gradually reduced during night. On the first day of weaning, during the 16-hour daytime period (06:00-22:00) maximum ventilation period allowed will be 8 hours accordingly to the treating physician’s judgment. During the 8-hour nighttime period (22:00-06:00), NIV should be used for at least 6 hours. From the second day of weaning, NIV duration will be gradually decreased during the day with steps of at least 2 hours/day at the discretion of the treating physician. Also, on the second day of weaning, discontinuation of overnight use will be considered, based on vital signs and ABG parameters recorded at 10 p.m. (see above), or a gradual decrease of at least 2 hours/night will be applied. When the patient continues to successfully pass the spontaneous breathing trial in the morning, and no criteria for NIV resumption (inclusion criteria) is met, with maximum NIV use of 4 hours during the 16-hours daytime, NIV may be permanently discontinued.
The integrated use of HFNC as a weaning technique from NIV is contemplated.
• HFNC: HFNC is initially set with airflow of 40 lpm titrated according to patient comfort and PaCO2, temperature of 34°C modulated according to patient comfort, FiO2 according to target SpO2 (88-92% in hypercapnic patients, 92-94% in hypoxemic patients), employing sized nasal cannulae which fit 50% of the nostril space. Treatment is continuous and weaning occurs when with airflow < 40 lpm and FiO2 < 50% at the breath test with O2 therapy, no criteria for resumption is met (similarly for what we have seen with NIV).
HFCWO arm and NIRT (interventional arm):
• NIRT delivered intermittently thrice a day (see above in the "NIRT Arm" section); In breaks from NIRT, HFCWO via The Vest® Airway Clearance System (HillRom, Batesville, Indiana, USA) is applied with vest placed around the chest for at least 15 minutes, thrice a day, setting frequency in the range of 13-15 Hz depending on the patient's tolerance level, setting pressure between 2 and 5 cm H2O so that the vest is tight to the chest but at the same time comfortable [22]. The device will be applied by a physiotherapist with expertise in RICU. During treatment, the patient should maintain a sitting position. Treatment with HFCWO can be discontinued when the patient is able to generate a PCEF>270 lpm or, in the case of insufficient co-operation to perform the maneuver, resumes to actively clear secretions either alone or with the help of an experienced physiotherapist/nurse or has a Cough score of 3 (clearance of 6 ml of sputum following three coughs).
After three treatment sessions with HFCWO, patient's satisfaction and degree of comfort are assessed by the following 6 items concerning The Vest® Airway Clearance System use: 1) convenient; 2) easy; 3) comfortable; 4) helpful to feel better; 5) helpful to breathe better; 6) safe feeling. Study participants are asked to use a 5-point scale (0 points: fully agree; 1 point: partially agree; 2 points: neither agree nor disagree; 3 points: partially disagree; 4 points: completely disagree) to report their degree of satisfaction (Likert scale). The total score is the sum of those obtained in each item and ranges from a minimum of 0 (maximum comfort) to a maximum of 24 (maximum discomfort).
All patients, both those in the control arm and in the interventional arm, will follow a physiotherapy program which consists of thrice a day sessions of secretions mobilization techniques such as:
• Active cycle of breathing techniques: Alternating execution of Breathing Control, Thoracic Expansion Exercise and Huffing.
Breathing Control: breathing performed at subject’s tidal volume and usual respiratory rate. Exhalation is not forced and the patient is asked to keep relaxed upper chest and shoulders while moving the lower chest and abdomen.
Thoracic Expansion Exercise: deep breaths with emphasis on the inspiratory phase and unforced exhalation. The purpose is to increase inspiratory capacity, reduce airway resistance and facilitate collateral ventilation.
Huffing: non-violent forced exhalation achieved by contracting the abdominal muscles and keeping the mouth and glottis wide open.
• TheraPEP device exercises: The exercises should be performed by the patient in a sitting position with the mouthpiece firmly held between the lips. The patient is asked to perform 8-10 active unforced breaths, trying to maintain an expiratory pressure of 10-20 cm H2O (indicated by the plunger in the device) starting from high lung volumes after an inspiratory act above tidal volume.
The ratio of inspiration to expiration time should be 1:3.
After performing the respirations, the patient should then perform 2-3 coughing maneuvers to expel any secretion present in the large airways. The cycle will be repeated 5 times with 1-2 minutes intervals.
Treatment sessions will be self-administered, demanded to patient education and supervised by physiotherapist or nurse.
Informed Consent
Informed consent acquisition is a multistep process:
1. A first conversation between doctor and patient to present the study will take place. It could be supported by any kind of document and/or tool useful to give the best information and to help the comprehension. Also, the informative material attached to the informed consent document can be used as supportive material.
2. Signing of the appropriate module of informed consent in the two sections:
a. Informational section related to protocol and human rights;
b. Informed Consent expression.
Concomitant therapies
Standard pharmacological treatment required during ARF is allowed. In the breaks free from NIRT conventional oxygen therapy is administered via Venturi mask or via nasal prongs at a FiO2 which allows to obtain a SpO2 target in the range of 88-92% for hypercapnic ARF and of 92-94% for hypoxemic ARF.
Case Report Form (CRF)
The following variables will be recorded at admission and during hospital stay:
• Demographics (age, sex, BMI, smoking history);
• Concomitant diseases: COPD, chest wall restriction, neuromuscular disorders, ILDs, chronic heart failure, obesity, primary or metastatic lung neoplasm;
• PFT 3 months prior to admission and 3 months after discharge (FEV1, FVC, FEV1/FVC, PEF, TLC, RV, DLCO) [optional];
• Numbers of hospitalizations in the previous year;
• Numbers of exacerbations in the previous year;
• Charlson Comorbidity index;
• Cause of admission: pulmonary disease exacerbation, upper airway infection, pneumonia, pleural effusion, cardiovascular failure, sepsis, post-operative ARF, cancer progression, trauma, drug abuse, acute or chronic kidney failure, other…;
• “Do not intubate” order: y/n;
• Date/time of RICU treatment beginning;
• Date/time of RICU treatment end;
• Date/time of NIRT beginning; ventilation setting (mode, PS, PEEP, RR, Vte);
• Vital signs (SpO2, systemic arterial pressure, RR, HR) monitor recorded; ABG values (pH, pCO2, pO2, HCO3-, SaO2) in room air/at a set FiO2 chosen at RICU admission and which allows to have a SpO2 between 88 and 92%; dyspnea Borg scale, Kelly-Matthay neurological index, RASS index: all these data will be reported on the CRF at RICU admission before randomization and 1h, 6h, 12h, 24h, 48h after NIRT start and after accordingly to clinical judgement in both arms;
• APACHE II score within 24 hours since RICU admission;
• Comfort during HFCWO application (comfort level assessed by Likert scale);
• Adverse events linked to NIRT use: even if
NIRT is generally life-saving, it carries the risk for some adverse events which can be harmful or increase mortality such as NIRT associated pneumonia (relatively rare and under-diagnosed) whose risk is considered to be proportional to treatment duration, even if this is not clearly demonstrated in literature because of the limited number of identified patients [28]. It has also been suggested that skin lesions in contact areas between skin and interface, even if a minor complication of NIRT, are more frequent with prolonged treatment [26]. Moreover, the sanitary expenditure linked to NIRT is considerably high, even if lower than the one for invasive mechanical ventilation;
• PCEF/Cough score/sputum volume;
• Need for and type of analog-sedation (y/n);
• Weaning from NIRT (y/n);
• NIRT failure for inability to manage secretions (y/n);
• NIRT failure for the need for bronchoscopy (y/n, how many);
• NIRT failure for the need for ETI/IMV (y/n);
• NIRT failure for death in patients with a DNI order;
• NIRT failure for other causes: gas exchange deterioration, ineffective analgo-sedation, hemodynamic instability not responsive to administration of an amine and fluid resuscitation, cardiac arrest, respiratory arrest, multi-organ failure, other…(specify);
• NIRT, HFNC and NIV treatment duration;
• RICU mortality (y/n);
• Hospital mortality (y/n);
• RICU and hospital stay duration;
• Discharge setting (home, health care residence; rehabilitation, other…);
• Respiratory support at discharge (conventional oxygen therapy, HFNC, NIV, mechanical ventilation via tracheostomy);
Post-discharge evaluation of PFTs / PCEF / 6MWT at 90 days [optional].
Statistical Analysis
Statistical analysis will be conducted using the analytic software SPSS, version 12.0 (SPSS Inc., Chicago, IL, USA). Continuous variables will be reported as mean value ± standard deviation (SD) or as median with interquartile range (IQR) (according to data distribution) and will be compared using Mann-Whitney U test or Kruskal-Wallis test. Chi-squared test will be used for categorical variables. A p-value inferior than 0.05 for a two tailed test will be regarded as statistically significant.
Good Clinical Pratice
This study will be conducted in accordance to Good Clinical Practice Principles and to National Law applying to Clinical Trial conduction. This protocol has already undergone local Ethics Committee approval by “Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana -Area Vasta Sud Est – address: Farmacia Ospedaliera AOUS - Viale Bracci, 16 - 53100 Siena telephone number: +39 0577-586358, e-mail address: c.etico@ao-siena.toscana.it” with protocol number “21987” on October 17th 2022.
Abbreviations
ABG: Arterial Blood Gas; ARF: Acute Respiratory Failure; BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease; CRF: Case Report Form; DLCO: Diffusing Lung Capacity For Carbon Monoxide; DNI: Do Not Intubate; ETI: Endotracheal Intubation; FEV1: Forced Expiratory Flow In The First Second; FIO2: Inhaled Fraction Of Oxygen; FVC: Forced Vital Capacity; HFCWO: High Frequency Chest Wall Oscillations; HFNC: High Flow Nasal Cannulae; HR: Heart Rate; ICU: Intensive Care Unit; ILDs: Interstitial Lung Diseases; IMV: Invasive Mechanical Ventilation; NIRT: Non Invasive Respiratory Therapy; NIV: Non Invasive Ventilation, P-A/C: Pressure-Assisted Controlled; PaCO2: Carbon Dioxide Arterial Pressure; PaO2: Oxygen Arterial Pressure; PCEF: Peak Cough Expiratory Flow; PFTS: Pulmonary Function Tests; PEEP: Positive End Expiratory Pressure; PS: Support Pressure; PSV: Pressure Support Ventilation; RASS: Richmond Analgo-Sedation Scale; RICU: Respiratory Intensive Care Unit; RR: Respiratory Rate, RV: Residual Volume; SaO2: Arterial Oxyhemoglobin Saturation; Sars-CoV-2: Severe Acute Respiratory Syndrome coronavirus 2; SpO2: Peripheral Oxyhemoglobin Saturation; TLC: Total Lung Capacity; Vte: Expiratory Tidal Volume.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. Muir JF, Cuvelier A, Verin E, et al. Noninvasive mechanical ventilation and acute respiratory failure: indications and limitations. Monaldi Arch Chest Dis. 1997; 52(1): 56-9.
2. Rochwerg B, Brochard L, Elliott MW. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. ERJ. 2017; 50(2): 1602426.
3.Oczkowski S, Ergan B, Bos L, et al. ERS Clinical Practice Guidelines: High-flow nasal cannula in acute respiratory failure. ERJ. 2021; 2101574.
4. Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. NEJM. 1995; 333(13): 817-22.
5. Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003; 326(7382): 185.
6.Meyer TJ, Hill NS. Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760-70.
7. Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. AMJRCC. 2012; 185(2): 152-9.
8. Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulm Med. 2014; 14: 19.
9. Scala R, Naldi M, Maccari U. Early fiberoptic bronchoscopy during non-invasive ventilation in patients with decompensated chronic obstructive pulmonary disease due to community-acquired-pneumonia. Crit Care. 2010; 14(2): R80.
10. Rochester C, Fairburn C, Crouch R, et al. Pulmonary rehabilitation for respiratory disorders other than chronic obstructive pulmonary disease. Clin Chest Med. 2014; 35(2): 369-89
11. Puhan M, Scharplatz M, Troosters T, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009; 21: CD005305.
12. Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008; 36(8): 2238–2243.
13. Nicolini A, Cardini F, Landucci N, et al. Effectiveness of treatment with high-frequency chest wall oscillation in patients with bronchiectasis. BMC Pulm Med. 2013; 13: 21.
14. Hansen LG, Warwick WJ, Hansen KL. Mucus transport mechanisms in relation to the effect of high frequency chest compression (HFCC) on mucus clearance. Pediatr Pulmonol. 1994; 17: 113–118.
15. Milla CE, Hansen LG, Weber A, et al. High-frequency chest compression: effect of the third generation compression waveform. Biomed Instrum Technol. 2004; 38: 322–328.
16. Fink JB, Mahlmeister MJ. High frequency oscillation of airway and chest wall. Respir Care. 2002; 47: 797-807.
17. Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol. 2007; 35: 116–129.
18.Kendrick AH. Airway clearance techniques in cystic fibrosis. ERJ. 2006; 27: 1082–1083.
19. Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc. 2004; 1: 42–46.
20. Lechtzin N, Wolfe LF, Frick KD. The impact of high-frequency chest wall oscillation on healthcare use in patients with neuromuscular diseases. Ann Am Thorac Soc. 2016; 13(6): 904–909.
21. Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne Muscular Dystro- phy. Chest. 1997; 112: 1024-28.
22. Chakravorty I, Chahal K, Austin G. A pilot study of the impact of high frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion. Int j COPD. 2011; 6: 693–699.
23. Renda T, Scala R, Corrado A, et al. Adult Pulmonary Intensive and Intermediate Care Units: The Italian Thoracic Society (ITS-AIPO) Position Paper. Respiration. 2021; 100: 1027- 1037.
24. Kelly BJ, Matthay HD. Prevalence and severity of neurological dysfunction in critically ill patients. Influence on need for continued mechanical ventilation. Chest. 1993; 104: 1818- 1824.
25. Graham J, Hall L, Gandevia B. Relationship of the loose cough sign to daily sputum volume: observer variation in its detection. Brit J Prev Soc Med. 1971; 25, 109-113.
26. Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet. 2009; 374: 250–9.
27. Boles J-M, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29: 1033–1056
28. Kohlenberg A, Schwab F, Behnke M, et al. Pneumonia associated with invasive and noninvasive ventilation: an analysis of the German nosocomial infection surveillance system database. Intensive Care Med. 2010; 36(6): 971-8.
Received:March 09, 2023;
Accepted: March 23, 2023;
Published: March 27, 2023.
To cite this article : : Cirilli L, Golfi N, Guidelli L, Renda T, Falcone ND, Tacconelli RTG, et al. The Role of High Frequency Chest Wall Oscillations Administered via the Vest® Airway Clearance System in Addition to Non-Invasive Respiratory Therapies in the Treatment of Patients with Acute Respiratory Failure and Hypersecretion: Monocentric, Parallel Group, Controlled Randomized Clinical Trial (VICOR). European Journal of Respiratory Medicine. 2023; 5(1): 377 - 383. doi: 10.31488/EJRM.139.
© The Author(s) 2023. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
