Research article/ Open Access
DOI:10.31488/EJRM.141
TLR4 Ligation by eNAMPT, a Novel DAMP, is Essential to Sulfur Mustard- Induced Inflammatory Lung Injury and Fibrosis
Carrie L. Kempf1, Jin H. Song1 , Saad Sammani1 , Tadeo Bermudez1 , Vivian Reyes Hernon1 , Lin Tang1, Hua Cai2 , Sara M. Camp1, Carly A. Johnson3 , Mohamed S. Basiouny3 , Leslie A. Bloomquist3 , Jacqueline S. Rioux3 , Carl W. White3 , Livia A. Veress3, Joe G. N. Garcia*1
1. Department of Medicine, University of Arizona Health Sciences, Tucson, AZ
2. Department of Anesthesiology, University of California Los Angeles, Los Angeles, CA
3. Department of Pediatrics, Center for Advanced Drug Development, University of Colorado Anschutz Campus, Aurora, CO
*Corresponding author: : Joe G.N. Garcia, MDUniversity of Florida UF Scripps Research Institute130 Scripps WayJupiter, FL 33458, USA.
Abstract
Objective:Human and preclinical studies of sulfur mustard (SM)-induced acute and chronic lung injuries highlight the role of unremitting inflammation. We assessed the utility of targeting the novel DAMP andTLR4 ligand, eNAMPT (extracellular nicotinamide phosphoribosyltransferase), utilizing a humanized mAb (ALT-100)in rat models of SM exposure.Methods:Acute(SM 4.2mg/kg, 24hrs), subacute (SM 0.8 mg/kg, day 7), subacute(SM 2.1mg/kg, day 14), and chronic(SM 1.2mg/kg, day 29) SMmodels were utilized.Results:Each SM model exhibitedsignificant increases in eNAMPTexpression(lung homogenates)and increased levels of phosphorylated NFkB and NOX4. Lung fibrosis (Trichrome staining) was observed in both sub-acute and chronic SM models in conjunction with elevated smooth muscle actin (SMA), TGFb,and IL-1b expression.SM-exposed rats receiving ALT-100 (1 or 4 mg/kg, weekly) exhibited increased survival, highly significant reductions in histologic/biochemical evidence of lung inflammation and fibrosis (Trichrome staining, decreasedpNFkB, SMA, TGFb, NOX4), decreased airways strictures, and decreased plasma cytokine levels (eNAMPT, IL-6, IL-1b.TNFa).Conclusion:The highly druggable,eNAMPT/TLR4 signalingpathway is a key contributor to SM-induced ROS production,inflammatorylung injury and fibrosis. The ALT-100 mAbis a potential medical countermeasureto address the unmet need to reduce SM-associated lung pathobiology/mortality.
Keywords: eNAMPT, DAMP, mAb, sulfur mustard
Introduction
Sulfur mustard is the most widely-used chemical weapon in history [1] and continues to exist as a significant threat. The pathobiology of SM-induced lung injuries is incompletely understood, however, a variety of human and preclinical studies of sulfur mustard (SM) exposure have highlighted the important role of unremitting inflammation in development of severe acute and chronic lung injuries events that are associated with excessive mortality[2-4]. Direct acute inhalational SM exposure initiates lung epithelial cell and endothelial cell cytotoxicity, increased vascular permeability, coagulation system activation with rapid deposition of fibrin plugs or casts that obstruct small and large airways, and acute respiratory distress syndrome (ARDS)events that all culminate in potentially fatal acuterespiratory failure[1]. The mechanisms underlying these events are currently postulated to be related to SM-induced tissue damage and release of “danger” signals or damage-associated molecular pattern molecules (DAMPs) which amplify inflammation via engagement with pathogen recognition receptors (PRR), including Toll-like receptor (TLR) family members[5-7]. DAMPs induce chemokine/cytokine release, with inflammatory cell tissue infiltration [5-6,8-9]that generate waves of ROS, and release of inflammatory cytokines, chemokines and growth factors. SM-mediated unchecked inflammation promotes acute pulmonary injury and SM-exposed survivors develop long-term complications including small airwaysclerosis known asbronchiolitis obliterans or BO, and parenchymal fibrosis[4]. While our published studies indicate fibrinolytic therapies are effective in reducingSM-induced acute airway obstruction [10-11], a critical unmet need exists for novel medical countermeasures (MCMs) to effectively mitigate SM-induced pulmonary and systemic inflammation, increased vascular permeability, BO and lung fibrosis, eventsthat drive SM mortality.
In prior work, we utilized genomic–intensive approaches to identify eNAMPT(extracellular nicotinamide phosphoribosyltransferase) as a novel DAMP and master regulator of evolutionarily-conserved inflammatory cascades via ligation of the Toll–like receptor 4 (TLR4). When secreted into the circulation, eNAMPTligates TLR4 [12-15] to elicit profound NFkB-driven inflammatory lung injury andprocesses involved in ARDS/VILI pathobiology[12]. We have shown that eNAMPT is a highly druggable innate immunity inflammatory target [12,16-20]whose plasma levels are linked to ARDS severity and mortality [21]. eNAMPT is encoded by NAMPT, a gene whose expression is induced by ARDS stimuli (hypoxia, trauma, infection, ventilator stress)[12,16,18-19,22-23]. NAMPT promoter SNPs, common in both Blacks and non-Hispanic Whites (NHWs), confer increased risk of ARDS severity and death [24]. We have shown that plasma eNAMPT levels and NAMPT lung tissue expression are increased in mice, rats, pigs, NHPs and humans in response to potentially lethal inflammatory stimuli including bacterial/viral infection, sepsis, hypoxia, ischemia/reperfusion, radiation and trauma [12,16-19,22-26]. Importantly, we have demonstrated in prior rodent LPS/VILI studies that eNAMPT is a highly druggable target that directly participates in ARDS/VILI pathobiology. ARDS/VILI-exposed mice and rats receiving a humanized eNAMPT-neutralizing mAb, ALT-100, exhibit profoundly reduced inflammatory lung injury and plasma cytokine levels[16,18]. Intracellularly, NAMPT is an enzyme (iNAMPT) that catalyzes nicotinamide adenine dinucleotide (NAD) synthesis[17,24,27]. iNAMPT secretion, however, increases circulating eNAMPT levels which we speculate is essential to SM-induced inflammatory injuries andmortality. As a novel DAMP,eNAMPT is master regulator of inflammatory cascades via TLR4 ligation [12].
This study was designed to examineeNAMPT as a novel MCM target driving SM-induced inflammatory lung injuries and lethality.We found that a single SM exposure acutely increases rat plasma eNAMPT levels and NAMPT lung tissue expression which is sustained chronically.Confirming eNAMPT/TLR4 as a highly druggable SM therapeutic target, IV-delivered eNAMPT-neutralizing ALT-100 mAb increased SM survival while reducing the severity of lung inflammation and injury (reduced airway strictures, fibrosis, plasma cytokines) in SM-exposed rats. Together, these studies directly address a critical unmet need for therapeutic drug development to reduce SM-induced pathobiology and mortality.The eNAMPT-neutralizing ALT-100 mAbappears to be a novel and effective medical countermeasure (MCM) to rescue SM-exposed subjects from acute and long-term pulmonary morbidity and death.
Materials and Methods
Reagents, chemicals and antibodies
Sulfur Mustard (SM; 2,20-dichlorodiethyl sulfide) was synthesized, characterized and validated at the University of Colorado Center for Advanced Drug Development (UC-CADD).eNAMPT-neutralizing humanized mAbs, ALT-100 was provided by Aqualung Therapeutics (Tucson, AZ, USA). Phospho-NF-kB p65 (Ser536) (93H1) Rabbit mAb were purchased from Cell Signaling Technologies (Danvers, MA). The NF-kB pAb was obtained from Invitrogen.Details of the eNAMPT-neutralizing polyclonal pAb and humanized mAb (ALT-100 mAb, Aqualung Therapeutics, Tucson, AZ) have been previously reported [16,18,28].
Ratmodels of sulfur mustard (SM) exposure
Two different exposure sites and models were used for these studies, one at Battelle Research Center (West Jefferson, Ohio), and the other at the UC-CADD in Denver, Colorado. UC-CADD’s SM exposure model used is as follows: Sprague-Dawley rats (male, 250 - 275g) were purchased from Charles River Laboratories (Raleigh, North Carolina), and were allowed 7 days to adjust to Denver altitude upon arrival at University of Colorado animal facility prior to use. A 12-hour light and dark cycle was maintained throughout housing, and food and water were provided ad libitum. The Institutional Animal Care and Use Committee of the University of Colorado approved these studies. We utilized well-established SM inhalation methodologies, with a rodent SM vapor inhalation system as previously described[29].Briefly, rats were anesthetized with ketamine (100 mg/kg)/xylazine (20 mg/kg) cocktail by intramuscular injection, intubated under direct visualization with a modified glass Pasteur pipette, and inhalation exposed to SM vapor for 50 minutes through a water jacketed chamber vaporizer. SM vapor doses of 4.2, 2.1 or 1.2 mg/kgwas used for exposures, calculated per each animal’s weight. At the end of the exposure, the endotracheal tube was removed, and the rats were returned to their cages for recovery. Animals were then administered a single dose of either IgG (4 mg/kg, IV) or ALT-100 (1 or 4 mg/kg, IV) [18]. Animals surviving to Day 14 and 29 were euthanized with phenotypic assessment of clinical, BAL, hemodynamic and biochemical indices.
In separate studies conducted at Battelle research laboratories, male rats ((Charles River Laboratories, 300-400g) were exposed to SM (0.8 mg/kg, via endotracheal tubes and temperature controlled vapor generator)and PBS or ALT-100 (1 or 4 mg/kg, IV) and examined for survival over a 7 day period.SM exposure and methodology was performed as previously described (30).
In addition to survival (using IACUC-approved Euthanasia Criteria), study endpoints included daily clinical observations of body weights, pulse oximetry, respiratory rates, Work of Breathing Score;and Activity Score. Euthanized rats at Day 14 were harvested for comparison of ALT-100 mAb-treated and IgG-treated SM-challenged rats. All in vivo studies were carried out in compliance with ARRIVE guidelines.
Animal monitoring and supplemental care for UC-CADDrat SM Inhalation model
were weighed and monitored for respiratory distress prior to exposure and then once daily until end of study at 28 days. A pulse oximeter (Starr Life Sciences, Oakmont, PA) was used to collect oxygen saturation (SpO2) daily, using an appropriately-sized neck collar in unanesthetized rats. SpO2 values were recorded pre-exposure, 4-hours after SM exposure, then daily for 5 days followed by twice weekly for 29 days. Rat weights (g) were recorded pre-exposure for baseline values, and then daily post-exposure. When an animal lost >20% total body weight, a sterile 0.9% NS subcutaneous fluid bolus was administered daily (10 ml/kg) as supportive care. Animals were euthanized when predetermined euthanasia criteria was met. Criteria for early euthanasia was defined as a 30% weight loss from an animal’s peak body weight, or an average pulse oximetry reading of <70% and a clinical score of ≥ 7 as previously described [29].
Lung specimen collection for UC-CADDrat SM inhalation model
At time of euthanasia, rats were anesthetized with a cocktail of ketamine (100 mg/ml)/xylazine (20 mg/ml)/acepromazine (10 mg/ml) via IP injection. Blood samples were collected through the descending aorta, followed by euthanasia via thoracotomy and exsanguination. Animals were then tracheally-cannulated and lungs were inflation-fixed as previously described (10) at 20 cm H2O with 4% paraformaldehyde (PFA) for 30 minutes, and then removed by gross dissection and placed in specimen container filled with 4% PFA for storage.
Quantitative histology and immunohistochemistry (IHC) analyses
For UC-CADD’s rat SM inhalation model, lungs were trimmed and processed on the TP1020 Benchtop Tissue Processor (Leica Biosystems, Buffalo Grove, IL). Histological stains hematoxylin and eosin and Masson’s trichrome were performed (Millipore Sigma-St. Louis, Missouri, Gill’s No. 3 Hematoxylin cat. #S232, Eosin Y solution, alcoholic cat. #HT110132, Trichrome Kit cat. #HT15).
For Battelle’s SM inhalation model, lung tissues were collected for histological assessment and fixed in 10% neutral buffered formalin for a minimum of 48 h, embedded in paraffin, sectioned, mounted onto slides, and stained with hematoxylin-eosin (H&E) or Trichrome blue. Routine H&E slides were prepared using Richard-Allan hematoxylin, clarifier, bluing reagent and eosin as we have previously described [16].
Histology samples of each group were randomly selected for quantification of H&E and Trichrome blue staining using ImageJ software [16,18] with images captured with light microscopy (Olympus digital camera) at magnification of 10x power and at different sections of each slide. For H&E and Trichrome blue image analysis an intensity adjustment approach was applied with auto image adjust setting selected and the percentage of area selected for measurement with all images processed and saved for statistical analysis.
Plasma biomarker measurements
A meso-scale ELISA platform was utilized (Meso Scale Diagnostics, Rockville, MD) for measurements of plasma levels of eNAMPT, IL-6, IL-8, and Angiopoetin-2. Each biotinylated antibody (10 µg/ml, specific for each analyte, was mixed with a different linker for each analyte and incubated for 30 min at 250C. The reaction was terminated with 200 µl of free biotin solution and 600 µl of the 10x U-PLEX linked biotinylated antibody solution with 50 µl of coating solution was added to each well in 96 well plate and incubated for 1 h (800 rpm shaking, 250C). After washing, each well was supplemented with 25 µl of diluent and 25 µl of calibrator or samples/standards, incubated for 1 h (800 rpm shaking, 2500C). After washing (TBS-T), each well was supplemented with 50 µl/well of 1x detection antibody solution, again incubated for 1h, washed and supplemented with 2x Read Buffer T followed by plate imaging and calculation of the absolute concentration values based on standards.
Western blotting and biochemical tissue analyses
Western blotting of lung tissue proteins was performed with densitometric analysis normalized to b-actin expression as previously reported[18]. Snap frozen lung and kidney tissues were homogenized in RIPA buffer (50 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, 0.5 % sodium deoxycholate, 0.1 % SDS, 1% NP-40, 5 mmol/L EDTA) supplemented with complete protease/phosphatase inhibitor cocktail (Cell Signaling Cat# 5872S) using tissue grinder with glass pestles (VWR Cat# 26307-606). After centrifugation (15,000 g for 20 min at 4°C), protein concentration of homogenates was determined by Bio-Rad DC protein assay (cat# 5000112). Following incubation 5 min at 90°C in loading buffer, aliquots containing equal amounts of protein (25–30 ug) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, proteins were transferred to PVDF membranes and probed with specific primary antibodies by horseradish peroxidase-conjugated secondary antibodies. Proteins were visualized using an ECL system (Pierce West Pico cat # 34580) and ChemiDoc MP imaging system (Bio-Rad). Densitometric analysis was performed using Bio-Rad Image Lab 6.01 software by normalizing the levels of proteins to b-actin expression. The levels of phosphor-proteins were quantified by normalizing the levels to their respective total proteins.
Statistical analysis
Continuous data were compared using nonparametric methods and categorical data by chi square test. Where applicable, standard one-way ANOVA was used and groups were compared using the Newman-Keuls test. Differences between groups were considered statistically significant when p values were less than 0.05 (p <0.05. T- test was used to compare the means of data from different experimental groups. If significant difference was present by T test (p <0.05), a least significant differences (LSD) test was performed post hoc. Between group differences were considered statistically significant when p values less than 0.05. Statistical tests were performed using GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.
Results
Effect of eNAMPT neutralization inan acute rat model of sulfur mustard-induced lung injury
Sprague-Dawley rats exposedto an acute SM exposure model(4.2 mg/kg,UC-CADD) exhibited 100% mortality at 24hr accompanied by histologic evidence of inflammatory lung tissue injury and leukocyte infiltration with prominent alveolar and bronchiolaredema(Figure 1A).In contrast,rats receiving the eNAMPT-neutralizing ALT-100 (1 mg/kg or 4 mg/kg)delivered 2 hrs post SM exposure exhibited 30%-40% survival (Figure 1B) and significantly reduced H&E evidence of acute SM-induced inflammatory injury compared with untreated SM-exposed rats(Figure 1A).In addition, rats receiving ALT-100 mAb, 1mg/kg or 4mg/kg,exhibited significantly reductions in theSM-mediated increases in NAMPT protein expression in lung tissuehomogenates (Figure 1C/D) as well as reduced levels of NOX4(Figure 1C/E) and phosphorylated NFkB (Figure 1F/G).
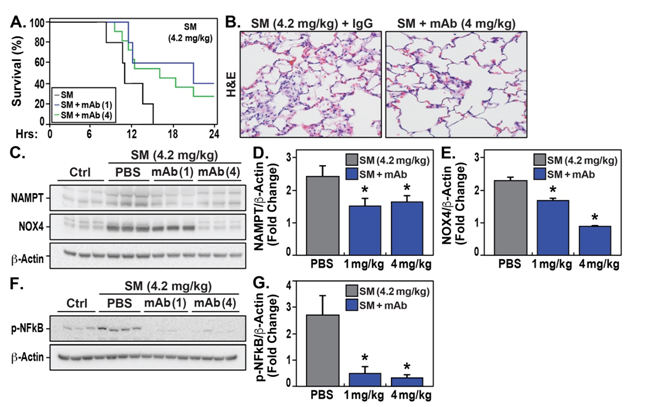
Figure 1:eNAMPT ALT-100 mAb reduces acute SM inflammatory injury. A. Shown is the 24 hour mortality in SM-exposed (4.2 mg/kg) rats. Rats treated with 1 and 4 mg/kg of ALT-100 mAb exhibited increased survivability beyond the 24 hours compared to untreated rats with 100% mortality. B. Depicted are representative H&E images from ALT-100 mAb treated (4mg/kg) (IV, n=5 all groups). vs untreated rats in the acute SM model (4.2 mg/kg, UC-CADD) C/D/E/F/G. Depicted are western blots of NAMPT, NOX4, and phosphorylated NFkB in lung tissue extracts (with b-actin as a loading control) with respective densitometry analysis. These studies show marked reductions in these inflammatory proteins in SM-exposed, ALT-100 mAb-treated rats.
Effect of eNAMPT neutralization in a sub-acute rat model of sulfur mustard-induced lung injury
We conducted a series of studies at two locations to evaluate the effects of the eNAMPT neutralization on sub-acute SM-induced lung injury. Two separate studies performed at Battelle Research Laboratories,rats were exposed to 0.8 mg/kgSM for3 hours and sacrificed at 7 days.Significant variability in mortalitywas observed in the two studies (70% survival vs 30% survival)resulting in a combined 45% survival. Rats receiving the ALT-100 mAb (n=5-10/group, 1mg/kg, 4mg/kg), delivered 2 hrs post SM exposure, ALT-100 demonstrated increased survival in the same 7 day survival compared to untreated rats with sub-acute SM exposure (50%/60% vs 45%) (Figure 2A).Macroscopic H&E staining of whole lung sections from ALT-100 (4mg/kg)-treated SM-exposed rats show a marked reduction in overall extent of inflammatory lung injury compared to untreated SM-exposed rats (Figure 2B), findings confirmed by microscopic H&E staininginALT-100 mA- treated animals (Figure 2C).

Figure 2:eNAMPT ALT-100 mAb reduces subacute SM inflammatory injury at day 7. A. Depicted is Kaplan-Meyer curve for 7-day mortality comprised of two rounds of sub-acute SM-exposed rat experiments (SM 0.8 mg/kg, Battelle Laboratories). SM-exposed rats treated with 1 and 4 mg/kg of ALT-100 mAb exhibited higher survivability at 7 days than untreated SM-exposed animals. B/C. Representative whole lung imaging and high magnification H&E images from sub-acute SM exposure studies at day 7 (SM 0.8 mg/kg) showing reduced SM-induced inflammatory cell infiltration and edema in rats receiving 4 mg/kg ALT-100 mAb .
A second series of subacute SM-induced lung injurystudies were performed at the University of Colorado (UC-CADD) withSD male rats exposed to 2.1 mg/kg and sacrificed at 14 days.In this UC-CADD model, SM-challenged ratsexhibitedonly 20% mortality at 14 days(5-10 rats/group) butdemonstrated highly significant histologic evidence of inflammation by H&E staining(Figure 3A). Lung micro-dissection atDay14 identified airway strictures in 100% of untreated SM-exposed rats (Figure 3B). Sub-acute SM-exposed rats receiving the eNAMPT-neutralizing ALT-100 mAbat either1 mg/kg or 4 mg/kgdemonstratedsignificant reduction inSM-induced histologic inflammation (Figure 3A, representative image shown withALT-1004 mg/kg) and prominent reductions in airway structures with both 1 mg/kg or 4 mg/kg as 40%-50% of ALT-100-treated ratswere completely free of airway strictures(Figure 3B). In addition, SM-exposed rats treated with IV ALT-100 4 mg/kg also showed increased functionality with elevated activity scores compared with untreated or 1mg/kg-treatedSM-induced rats (Figure 3C, red line).

Figure 3:eNAMPT ALT-100 mAb reduces sub-acute SM inflammatory injury at day 14. A. Representative H&E images from sub-acute SM studies (SM 2.1 mg/kg, UC-CADD) from 14 days post-exposure, showing mild SM-induced inflammatory cell infiltration and edema in survivors which was markedly reduced in rats receiving 4 mg/kg ALT-100 mAb. B. Quantification of airway strictures (left lobe) at 14 days in sub-acutely-exposed SM rats (2.1 mg/kg, UC-CADD) demonstrate 100% of untreated rats with strictures whereas 40%-50% of 1 mg/kg or 4 mg/kg ALT-100 mAb-treated rats (IV on Days 1 and 7) were completely free of any airway strictures. C. Functional activity scores show sub-acute SM-exposed rats (2.1 mg/kg, UC-CADD) treated with IV ALT-100 4 mg/kg with increased activity scores Days 6-12 compared with untreated rats or 1mg-treated rats. D/E/F. Sub-acute SM-exposed rats receiving ALT-100 mAb (1 or 4 mg/kg) show reductions in SM-induced increases in NAMPT tissue expression at 14 days, detected by western blotting of SM-exposed lung tissue homogenate with densitometry. F. Circulating plasma levels of eNAMPT at are increased in SM-exposed animals at 14 days, but reduced in rats treated with the ALT-100 mAb (1 or 4 mg/kg).
Biochemical studies of lung tissues and plasma measurements from ALT-100-treated rats (1 mg/kg or 4 mg/kg) in the UC-CADD subacute 2.1 mg/kg SM model strongly support the protective effects of targeting eNAMPT/TLR4 signaling as both ALT-100 mAbdoses produced prominent inhibition of SM-induced increases in NAMPT expression in tissue homogenates (Figure 3D/E) andreductions in SM-induced increases in plasma eNAMPT levelsat 14 days post-exposure (Figure 3F). The ALT-100 mAb exerted similar protective effects when biochemical indices of lung inflammation and fibrosis were assessed,as mAb-treated rats showed reductions in SM-induced NFkB activation (phosphorylation)(Figure 4A/B),and JNK MAP kinase activation (Figure 4A/C) at 14 days. SM-mediated increases in indices of lung fibrosis at Day 14 including increased expression of smooth muscle actin (SMA) (Figure 4D/E), TGFb (Figure 4D/F), and the ROS-producing enzyme, NOX4(Figure 4D/G).Each index of fibrosis was attenuated in rats receiving ALT-100 mAb with 4mg/kg significantly more effective than 1mg/kg.Thus, although lethality was quite modest in the UC-CADD sub-acute model of SM exposure, the eNAMPT-neutralizing ALT-100 mAbappears to reduce SM-induced rat lethalityand histologic and biochemical indices of inflammation and fibrosis.
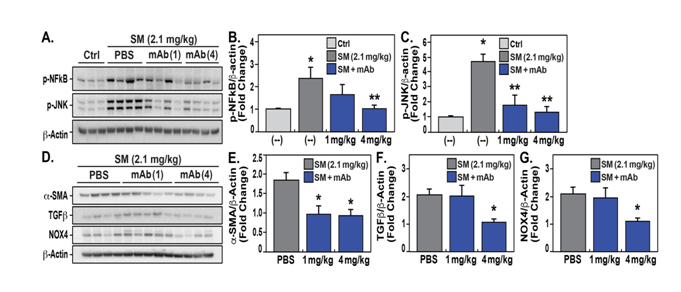
Figure 4:ALT-100 mAb reduces subacute SM inflammatory lung injury and expression of inflammatory proteins at day 14. A/B/C. Expression levels of p-NFkB and p-JNK detected by Western blots in lung homogenates from 2.1mg/kg SM-exposed rats on day 14 show significant increases in each protein with significant reduction in expression levels in ALT-100 mAb-treated rats compared to untreated rats with quantification by densitometry. D/E/F/G. Expression levels of smooth muscle actin (SMA), TGFβ, and NOX4 in lung homogenates are significantly increased in rats exposed to 2.1mg/kg SM on day 14. Each index of lung fibrosis was significantly reduced in rats receiving the ALT-100 mAb. For each protein assessed, ALT-100 mAb at 4mg/kg provided significantly greater protection than 1mg/kg
Effect of eNAMPT neutralization in a low-doserat model of sulfur mustard-induced chronic lung injury
A SM exposure model (1.2 mg/kg, UC-CADD)showed that rats sacrificed at day 29demonstrated histologic evidence of inflammatory lung injurywith significantly increased Trichrome staining consistent with lung fibrosis quantified by Image J analysis (Figure 5A/B).While mortality was only 20% in this SM model of chronic lung injury, ALT-100-treated rats (1mg/kg weekly IV) exhibited 100% survival with reduced Trichrome staining, and reduced lung tissue expression of NAMPT, smooth muscle actin (SMA),TGFβand NOX4(Figure 5C/D/E/F/G) at 29 days. SM exposure inducedincreases in plasma levels of IL-1b, IL-6, and TNFawhich were attenuated in mAb-treated rats(Figure 5H/I/J).These studies are highly consistent with a critical role for the eNAMPT/TLR4 signaling pathway in SM-induced inflammatory cascade activation and fibrosis with ALT-100 mAbas a viable MCMto reduce SM severity and lethality.

Figure 5:ALT-100 mAb reduces chronic SM inflammatory lung injury and fibrosis at day 29. A/B. Trichrome blue lung staining and ImageJ analysis of fibrotic lung tissue in the chronic SM model (1.2 mg/kg UC-CADD, 29 days). SM-exposed rats exhibited marked increases in Trichrome expression, consistent with significant lung fibrosis, when compared to controls. ALT-100 mAb-treated SM rats (1 mg/kg, 1x weekly) demonstrated significant reductions in the extent of Trichrome staining at Day 29 (ImageJ quantification). C/D/E/F/G. Western blots and densitometry of 29 day SM-exposed lung tissues show increased NAMPT, SMA, NOX4 and TGFβ immunoreactivity compared to controls, which is reduced by ALT-100 mAb treatment (captured by densitometry). H/I/J. SM exposure increases plasma levels of IL-6, IL-1b, and TNFa at 29 days, with ALT-100 mAb-treated rats (1mg/kg, 1x weekly) showing reduced levels.
Discussion
The current study was designed to directly address the unmet need for a MCMfor subjects exposed to sulfur mustard (SM) who are at risk for SM-induced acute and chronic lung injuries and toxicities. Our data confirm human and preclinical studies that have highlighted the role of unremitting inflammation in the development of SM-induced severe lung injuries [6,8]and strongly implicate eNAMPTas an immune effector molecule anddamage-associated molecular pattern protein (DAMP)that is released by SM-induced tissue damage(Figure 6). We have shown that eNAMPTcontributes to SM-induced TLR4 inflammatory cascades to increase lung vascular permeability and edema, activatethe coagulation cascade resulting in acute lethality. Utilizing the humanized eNAMPT-neutralizing ALT-100 mAb (1 or 4 mg/kg, weekly), we demonstrated that ALT-100 mAbdampens the SM-induced lung inflammatory response and reduces SM-induced lung injury and fibrosis, a strategy to potentially improve survival following lethal SM exposure[7-8].
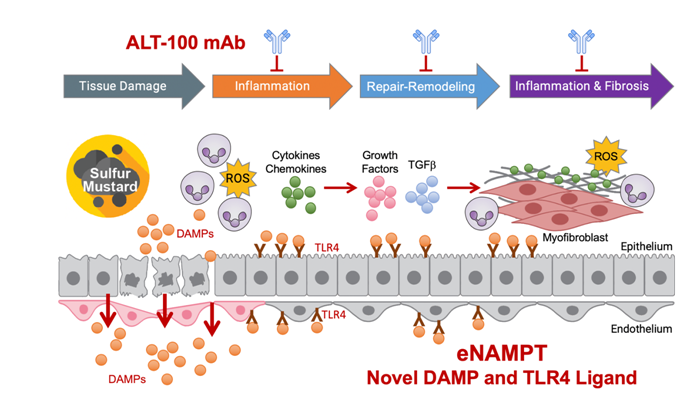
Figure 6:Timeline of lung tissue responses to SM exposure. Directlung exposure to SM results in endothelial/epithelial tissue damage and release ofmultiple DAMPs including eNAMPT.SM-mediated eNAMPT release and subsequent activation of the TLR4 inflammatory cascade, results in chemokine/cytokine release, inflammatory cell tissue infiltration,increased permeability, activation of coagulation cascades resulting in fibrin casts and airway obstruction, and potentially fatal respiratory failure. In surviving SM-exposed subjects, chronic inflammation ensues which is driven by sustained eNAMPT secretion and eNAMPT/TLR4 profibroticsignaling leading to dysregulated tissue repair and remodeling facilitated by TGFβ. The extent of resulting small airway sclerosis (bronchiolitis obliterans) and lung fibrosis is a major determinant of SM-induced mortality. Figure adapted from [35].
The involvement of eNAMPT/TLR4 inflammatory signaling in SM-mediated chemical injury is completely consistent with the role of this target in acute inflammatory lung injuries such as trauma-, pancreatitis-, sepsis-, and COVID-19-induced ARDS as well in subacuteradiation pneumonitis. NAMPT was the top dysregulated gene in our reported preclinical ARDS/VILI studies in C57BL6 mice, Sprague-Dawley rats, and Yucatan minipigs and dogs exposed to LPS, and to high tidal volume mechanical ventilation(VILI)[12,16-17,20,24]with increased IHC NAMPT staining, NAMPT protein expression in lung homogenates, and increased plasma eNAMPT levels, a finding consistent with SM-challenged rats in the current study [16,18-19]. In each preclinical mode of ARDS/VILI, model, we observed significant increasesin the levels of phosphorylated NFkB and NOX4 in lung tissue homogenates which were attenuated in animals receivingthe ALT-100 mAb[16,18-19].Although the protective effect of ALT-100 mAb on SM-induced lung injury were less impressive than efficacy in LPS/VILI models of lung injury, possibly reflecting the elaboration of multiple DAMPs in SM-injured tissues, treatment with the ALT-100 mAb did serve to reduce the severity of histologic inflammation and injury, reduce ROS production (via reduced NOX4 expression),TLR4 inflammatory cascade activation,BAL total PMNs, airway strictures and plasma levels of several key inflammatory biomarkers (eNAMPT, IL-6, Ang-2)[16]. These studies provide additional support for the premise that the eNAMPT/TLR4 inflammatory signaling pathways is a major driver of lung injury in SM-induced lung injury and fibrosis.
In addition to serving as a potent anti-inflammatory biologic, our data in SM-exposed rats treated with the eNAMPT-neutralizing ALT-100 mAb support increasing evidence for the ALT-100 mAbto serve as an effective anti-fibrotic biologic therapy with significant reductions inlung parenchymal fibrosis (Trichrome staining) and reductions in airway strictures in SM-exposed rats at 14 and 29 days post SM(SMA, TGFb). We previously reported elevated lung tissue NAMPT expression in whole thoracic irradiation-exposedC57Bl6 mice and Macaca mulatta NHPs with significant lung fibrosis at 12 weeks [25]. WTLI-exposed mice that received the eNAMPT ALT-100 mAb exhibited significant reductions in Trichrome staining, and lung tissue levels of smooth muscle actin, TGFb expression and SMAD signaling. The protective anti-fibrotic effects of the eNAMPTmAbare not lung-specific as reductions in hepatic fibrosis were observed in mice with established non-alcoholic steatosis who were begun on the eNAMPTmAb[31]. Similarly, eNAMPTmAb-treated rats with ischemia-induced cardiac fibrosis exhibited similar reductions in cardiac collagen and fibrosis [32].The eNAMPT-neutralizingmAbalso reduced eNAMPT/TLR4-mediated inflammatory signaling in the pathobiology of systemic lupus erythematosus pulmonary vasculitis and alveolar hemorrhage [33]. Finally, colon fibrosis in a murine model of ulcerative colitis was attenuated by eNAMPT neutralization [34]. Together, these studies validate eNAMPT/TLR4 signaling as a key contributor to SM-induced chronic inflammation and dysregulated tissue repair leading to small airway sclerosis (bronchiolitis obliterans) and lung fibrosis and support ALT-100 mAb as an anti-fibrotic therapeutic intervention.
Supporting the feasibility of ALT-100 mAb as a SM MCM, we have completed pharmacokinetic (PK) studies in rats and minipigs demonstrating that IV-delivered ALT-100 mAb exhibits a T1/2 half-life of 8-10 days in rats and 21-30 days in pigs. Our IND-enabling toxicity studies in rats and minipigs failed to identify discernable toxicity (28 day study) even with ALT-100 mAb doses of 50 mg/kg, 50-100 times the therapeutic dose of ALT-100 mAb. Importantly, we have completed ALT-100 mAb stable cell line development and chemical manufacturing (CMC) studies with a 200L GMP Bioreactor run yielding expression at 6 gms/L allowing for the generation of 3500 clinical ALT-100 doses (10mg/mL in 10 mL vials). We are currently utilizing these ALT-100 mAb doses for a Phase 1A safety/pharmacokinetic study in healthy human volunteers. Sufficient mAb is available for Phase 2A and Phase 3 clinical trials for an ARDS indication and an IND application has been submitted for the indication of ARDS (August 2022). Future studies will assess subcutaneous delivery of ALT-100 mAbin subacute and chronic SM rat models to allow for progress toward the Animal rule for approval as a SM MCM.
Despite the high novelty of the current study, several limitations should be noted. One important issue is the small sample size for a number of the study groups thereby limiting strong validation of the findings obtained. A second limitation is the lack of corroborative complementary genomic indices in SM-exposed tissues that show the activation of evolutionarily-conserved inflammatory cascades with a mitigating effect of the eNAMPT-neutralizing mAb. Finally, we employed IV administered mAb in our SM exposure rat studies. For ALT-100 to serve as a bona fide medical counter-measure requires an orally- or subcutaneously- delivered therapeutic. In this regard, recent data in preclinical models of lupus vasculitis and radiation-induced organ injury indicate that subQ-delivered mAb is highly effective in attenuating inflammatory injuries.
Conclusions
In summary,utilizingwell-established acute, subacute and chronic rat models of SM-induced inflammatory lung injury, we have demonstrated thatthe novel DAMP,eNAMPT,and the eNAMPT/TLR4 inflammatory cascade, arehighly druggable SM targets.The eNAMPT-neutralizing humanized mAbALT-100,proved to be aneffective therapeutic strategy to attenuate SM-inducedinflammatory lung injury and fibrosis and lethality.Further studies are needed to optimize ALT-100 mAb treatment of SM lung injuries, i.e. to determine efficacy via the subcutaneous route with optimized effective concentration. Additionally, potential synergies with other proposed SM MCMs via multi-prong therapeutic approaches directed at both acute (airway coagulation, etc), and chronic processes (BO, parenchymal fibrosis) can be explored. Nevertheless, ALT-100 mAbappears to serve as a strong medical countermeasure (MCM) candidate to address the unmet need for novel therapeutic strategiesto reduce SM-associatedlung pathobiology and mortality.
Abbreviations
eNAMPT: extracellular nicotinamide phosphoribosyltransferase;DAMP: damage-associated molecular pattern protein;mAb: monoclonal antibody;TLR4: Toll-like receptor 4
References
1. Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, et al. An Official American Thoracic Society Workshop Report: Chemical Inhalational Disasters. Biology of Lung Injury, Development of Novel Therapeutics, and Medical Preparedness. Ann Am Thorac Soc. 2017; 14:1060-1072. PMID: 28418689; PMCID: PMC5529138.
2. Aghanouri R, Ghanei M, Aslani J, Keivani-Amine H, Rastegar F, Karkhane A. Fibrogenic cytokine levels in bronchoalveolar lavage aspirates 15 years after exposure to sulfur mustard. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L1160-1164. PMID: 15286001.
3. Emad A, Emad Y. Levels of cytokine in bronchoalveolar lavage (BAL) fluid in patients with pulmonary fibrosis due to sulfur mustard gas inhalation. J Interferon Cytokine Res. 2007; 27:38-43. PMID: 17266442.
4. Weinberger B, Laskin JD, Sunil VR, Sinko PJ, Heck DE, Laskin DL. Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity. PulmPharmacol Ther.2011; 24:92-99. 2011. PMID: 20851203; PMCID: PMC3034290.
5. Chehardoli B, Nadi M, Khamis Abadi A, Kia A, Shahriary A, Salimian J. Immunomodulatory Effect of Curcumin in the Upregulation of Inflammasome Pathway Genes Induced by Sulfur Mustard Analog: An In-vitro Study. Iran J Allergy Asthma Immunol. 2011; 20:169-177. PMID: 33904675.
6. Malaviya R, Bellomo A, Abramova E, Croutch CR, Roseman J, Tuttle R, et al. Pulmonary injury and oxidative stress in rats induced by inhaled sulfur mustard is ameliorated by anti-tumor necrosis factor-alpha antibody. Toxicol Appl Pharmacol. 2021; 428:115677. PMID: 34390737.
7. Menendez D, Shatz M, Azzam K, Garantziotis S, Fessler MB, Resnick MA. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 2011; 7: e1001360. PMID: 21483755; PMCID: PMC3069118.
8. Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, Conti ML, et al. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol Appl Pharmacol. 2010; 248:89-99. PMID: 20659490; PMCID: PMC3954123.
9. Tahmasbpour E, Reza Emami S, Ghanei M, Panahi Y. Role of oxidative stress in sulfur mustard-induced pulmonary injury and antioxidant protection. Inhal Toxicol.2015; 27: 659-672. PMID: 26446928.
10. McGraw MD, Dysart MM, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, et al. Bronchiolitis Obliterans and Pulmonary Fibrosis after Sulfur Mustard Inhalation in Rats. Am J Respir Cell Mol Biol.2018; 58:696-705. PMID: 29314868; PMCID: PMC6002659.
11. McGraw MD, Osborne CM, Mastej EJ, Di Paola JA, Anderson DR, Holmes WW, et al. Editor's Highlight: Pulmonary Vascular Thrombosis in Rats Exposed to Inhaled Sulfur Mustard. Toxicol Sci. 2017; 159:461-469. PMID: 28962529; PMCID: PMC5837673.
12. Camp SM, Ceco E, Evenoski CL, Danilov SM, Zhou T, Chiang ET, et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFkappaB Signaling and Inflammatory Lung Injury. Sci Rep. 2015; 5:13135. PMID: 26272519.
13. Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999; 189:1777-1782. PMID: 10359581. PMCID: 2193086.
14. Viriyakosol S, Kirkland T, Soldau K, Tobias P. MD-2 binds to bacterial lipopolysaccharide. J Endotoxin Res. 2000; 6:489-491. PMID: 11521076.
15. Wolfson RK, Mapes B, Garcia JG. Excessive mechanical stress increases HMGB1 expression in human lung microvascular endothelial cells via STAT3. Microvasc Res. 2014; 92:50-55. PMID: 24370952. PMCID: 4327945.
16. Bermudez T, Sammani S, Song JH, Reyes Hernon V, Kempf CL, Garcia AN, et al. eNAMPT neutralization reduces preclinical ARDS severity via rectified NFkB and Akt/mTORC2 signaling. Sci Rep. 2022; 12:696. PMID: 35027578. PMCID: PMC8758770.
17. Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008; 178:605-617. PMID: 18658108.
18. Quijada H, Bermudez T, Kempf CL, Valera DG, Garcia AN, Camp SM, et al. Endothelial eNAMPT Amplifies Preclinical Acute Lung Injury: Efficacy of an eNAMPT-NeutralisingmAb. Eur Respir J. 2021; 57:2002536. PMID: 33243842. PMCID: PMC8100338.
19. Sammani S, Bermudez T, Kempf CL, Song JH, Fleming JC, Reyes Hernon V, et al. eNAMPT Neutralization Preserves Lung Fluid Balance and Reduces Acute Renal Injury in Porcine Sepsis/VILI-Induced Inflammatory Lung Injury. Front Physiol. 2022; 13:916159. PMID: 35812318; PMCID: PMC9257134.
20. Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005; 171:361-370.PMID: 15579727.
21. Bime C, Casanova N, Oita RC, Ndukum J, Lynn H, Camp SM, et al. Development of a biomarker mortality risk model in acute respiratory distress syndrome. Crit Care. 2019; 23:410. PMID: 31842964; PMCID: PMC6916252.
22. Ahmed M, Zaghloul N, Zimmerman P, Casanova NG, Sun X, Reyes Hernon V et al. Endothelial eNAMPT drives EndMT and preclinical PH: Rescue by an eNAMPT-neutralizing mAb. Pulm Circ. 2021; 11:20458940211059712. PMID: 34790349. PMCID: PMC8591779.
23. Chen J, Sysol JR, Singla S, Zhao S, Yamamura A, Valdez-Jasso D, et al. Nicotinamide Phosphoribosyltransferase Promotes Pulmonary Vascular Remodeling and Is a Therapeutic Target in Pulmonary Arterial Hypertension. Circulation. 2017; 135:1532-1546. PMID: 28202489; PMCID: PMC5400780.
24. Sun X, Elangovan VR, Mapes B, Camp SM, Sammani S, Saadat L, et al. The NAMPT promoter is regulated by mechanical stress, signal transducer and activator of transcription 5, and acute respiratory distress syndrome-associated genetic variants. Am J Respir Cell Mol Biol. 2014; 51:660-667. PMID: 24821571. PMCID: 4224084.
25. Garcia AN, Casanova NG, Kempf CL, Bermudez T, Valera DG, Song JH, et al. eNAMPT is a Novel DAMP that Contributes to the Severity of Radiation-Induced Lung Fibrosis. Am J Respir Cell Mol Biol. 2022; 66:497-509. PMID: 35167418. PMCID: PMC9116358
26. Garcia AN, Casanova NG, Valera DG, Sun X, Song JH, Kempf CL, et al. Involvement of eNAMPT/TLR4 signaling in murine radiation pneumonitis: protection by eNAMPT neutralization. Transl Res. 2021; 239:44-57. PMID: 34139379. PMCID: PMC8671169.
27. Wang T, Zhang X, Bheda P, Revollo JR, Imai S-i, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nature Structural & Molecular Biology. 2006; 13:661.
28. Sun BL, Tang L, Sun X, Garcia AN, Camp SM, Posadas E, et al. A Humanized Monoclonal Antibody Targeting Extracellular Nicotinamide Phosphoribosyltransferase Prevents Aggressive Prostate Cancer Progression. Pharmaceuticals (Basel).2021; 14. PMID: 34959723, PMCID: PMC8706080.
29. Veress LA, Anderson DR, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, et al. Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. Toxicol Sci. 2015; 143:178-184. PMID: 25331496; PMCID: PMC4274387.
30. Perry MR, Neal M, Hawks R, Pressburger D, Satola J, Triplett C, et al. A novel sulfur mustard (HD) vapor inhalation exposure model of pulmonary toxicity for the efficacy evaluation of candidate medical countermeasures. InhalToxicol. 2021; 33:221-233. PMID: 34396872. PMCID: PMC8602763.
31. Sun BL, Sun X, Kempf CL, Song JH, Casanova NG, Camp SM, et al. Involvement of eNAMPT/TLR4 inflammatory signaling in progression of non-alcoholic fatty liver disease, steatohepatitis, and fibrosis. FASEB J. 2023; 37:e22825. PMID: 36809677.
32. Liu Z, Sammani S, Barber CJ, Strom J, Kempf CL, Li F, et al. An eNAMPT-neutralizing mAb reduces post-infarct myocardial fibrosis and left ventricular dysfunction. JACC: Basic to Transl Sci. 2023 (submitted).
33. Tumurkhuu G, Casanova NG, Kempf CL, Ercan Laguna D, Camp SM, Dagvadorj J, et al. eNAMPT/TLR4 inflammatory cascade activation is a key contributor to SLE Lung vasculitis and alveolar hemorrhage. J TranslAutoimmun. 2023; 6:100181. PMID: 36619655, PMCID: PMC9816774.
34. Colombo G, Clemente N, Zito A, Bracci C, Colombo FS, Sangaletti S, et al. Neutralization of extracellular NAMPT (nicotinamide phosphoribosyltransferase) ameliorates experimental murine colitis. J Mol Med (Berl). 2020; 98:595-612. PMID: 32338310.
35. Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2021; PMID: 34211187.
Received:December 12, 2023;
Accepted: January 02, 2024;
Published: January 08, 2024.
To cite this article : Kempf CL, Song JH, Sammani S, Bermudez T, Hernon VR, Tang L, et al. TLR4 Ligation by eNAMPT, a Novel DAMP, is Essential to Sulfur Mustard- Induced Inflammatory Lung Injury and Fibrosis. European Journal of Respiratory Medicine. 2024; 6(1): 389 - 397. doi: 10.31488/EJRM.141.
© The Author(s) 2024
