Research article/ Open Access
DOI:10.31488/EJRM.135
Usefulness of Chest CT Scan in Haematological Patients with Severe Acute Respiratory Failure and Respiratory Virus Detection
Muriel Picard#1, Amazigh Aguersif#1, Emilie Bérard2, Audrey Fernandez2, Alexandre Nguyen3, Marion Jaffro3, Sihem Bouharaoua1, Jean Michel Mansuy4, Catherine Mengelle4, Guillaume Morel5, Cyril Cadoz6, Laura Platon7, Alexis Ferre8, Camille Verlhac9, Nahema Issa10, Laurent Argaud11, Guillaume Geri12, Danielle Reuter13, Guillaume Ducos1, Véronique Ramonda1, Jean Ruiz1, Christian Récher14, Elie Azoulay15, Stein Silva*1
1. Intensive Care Unit, Institut Universitaire du Cancer de Toulouse-Oncopole, University Teaching Hospital of Toulouse, France
2. Department of Epidemiology, Toulouse University Hospital, Toulouse, France. CERPOP, Université de Toulouse, Inserm, UPS, Toulouse, France
3. Department of Radiology, Rangueil Teaching Hospital, Toulouse, France
4. Laboratory of Virology, Purpan Teaching Hospital, Toulouse, France
5. Medical Intensive Care Unit, Hautepierre Teaching Hospital, Strasbourg, France
6. Medical-Surgical Intensive Care Unit, Mercy Teaching Hospital, Metz, France
7. Medical Intensive Care Unit, Lapeyronie Teaching Hospital, Montpellier, France
8. Medical Intensive Care Unit, André Mignot Hospital, Versailles, France
9. Medical-Surgical Intensive Care Unit, Estaing Teaching Hospital, Clermont-Ferrand, France
10. Medical Intensive Care Unit, Pellegrin Teaching Hospital, Bordeaux, France
11. Medical Intensive Care Unit, Edouard Herriot Teaching Hospital, Lyon, France
12. Medical-Surgical Intensive Care Unit, Ambroise Paré Hospital, AP-HP, Paris, France
13. Medical-Surgical Intensive Care Unit, Sud Francilien Hospital, Corbeil-Essonnes, France
14. Department of Hematology, Institut Universitaire du Cancer de Toulouse -Oncopole, University Teaching Hospital of Purpan, Toulouse, France. Université Toulouse III Paul Sabatier, Toulouse, France
15. Medical Intensive Care Unit, Hôpital Saint-Louis, AP-HP. Nord-Université de Paris
*Corresponding author: Pr. Stein SILVA (M.D., Ph.D.), Critical Care Unit, Institut Universitaire du Cancer de Toulouse-Oncopole, University Hospital of Purpan, Toulouse 31300, France, ToNIC lab, INSERM1214, Tel: +33(0)561772288; Fax: +33(0)561772289
#both authors contributed equally
Abstract
Chest CT scan is frequently used to provide information about respiratory system structural integrity in critically ill haematological patients with acute respiratory failure (ARF) and respiratory virus detection. However, there is only low-level evidence to recommend use of this radiological assessment. To fill this knowledge gap, we retrospectively ssessed prospectively collected CT scan, clinical and virology data from 101 severely ill haematological patients with ARF and respiratory virus detection in ten University affiliated centers in France between January 2008 and April 2018. This study was approved by the Ethic Committee of University Teaching Hospital of Toulouse. First, we observed that chest CT scan patterns allow differentiating virus-only pneumonia from co-infections. Second, among virus-only infection patients, the broncho-alveolar radiological feature was more frequently observed in patients infected by Orthomyxovirus (p < 0.007). Finally, in multivariate analysis, aggregate lesion intensity score (i.e. number of lung lobes) was significantly and independently associated with mechanical ventilation and ICU mortality (OR =1.88 for each lobe with lesion [95% CI: 1.27; 2.80], p = 0.002 and OR =1.64 [95% CI: 1.10; 2.44], p = 0.002; respectively), with a cumulative effect based on the number of affected lobes. The poor outcome that we report, highlight the need of further research to design more personalized intervention strategies for these patients, which will be at least in part be based upon chest CT scan data.
Key words: Hematological malignancies, respiratory virus, acute respiratory distress syndrome, intensive care unit, invasive mechanical ventilation, radiological findings, aggregate lesion intensity
Introduction
Acute respiratory failure (ARF) is the leading cause of intensive care unit (ICU) admission in immunocompromised patients [1] and is associated to a very high risk of mortality [2-3]. Early identification of the cause of ARF is associated with improved outcomes [4]. Therefore, identifying ARF aetiology while optimizing patient’s oxygenation and providing appropriate organ support at the same time, are cornerstones for the initial management of these patients. ARF can have various aetiologies, but pulmonary infection and its sequelae remain the most frequent precipitants in those that require ICU. Among the different infectious agents which cause pulmonary infection in immunocompromised patients, pneumonia caused by respiratory virus has been associated with a particularly high mortality rate [3-5].
Discriminating the viral ARF cause in critically ill haematological patients remains problematic due to nonspecific clinical presentation which has considerable overlaps among them [6]. Current strategies to diagnosed viral ARF aetiology in these patients are based on PCR assays on airway samples [7-8]. A broad use of polymerase chain reaction (PCR) methods has permitted to increasingly recognised viral pathogen in this clinically challenging setting [9, 10]. However, the clinical significance of respiratory virus detection in critically ill haematology patients is a still moot issue. A positive virus PCR test on upper airway samples might reflect either upper/lower respiratory tract infection, or also indicate asymptomatic carriage, perhaps with an increased viral burden because of worsening of immunosuppression [11].
Although there is only low-level evidence to recommend use of radiological assessments in this context, computerized tomography (CT) scan is frequently used to provide highly needed information about respiratory system structural integrity. Nevertheless, data on the radiological findings of the individual viruses are very limited and the frequency of particular CT scan patterns in favour of bronchial/bronchiolar (bronchial wall thickening, tree-in bud opacities), parenchymal (multifocal consolidations) and interstitial (ground-glass opacities, reticulations, honeycombing) lung anomalies and their associations with specific respiratory virus in critically ill immunocompromised patients have not yet been described [12-14]. It is worth noting that the radiological appearances of respiratory virus on immunocompromised patients are unclear, because they may be accompanied by other bacteria or fungi pathogens [15], and there is no data on the usefulness of CT scan to disentangle between these aetiologies. Finally, despite the promise hold by CT scan use for risk stratification of critically ill haematological patients with ARF and detected respiratory virus, we lack data on the value of early acquired chest CT scan to predict relevant outcomes, both in terms of need of invasive respiratory support need and ICU mortality.
The global objective of this study was to assess the clinical relevance of chest CT scan for the management of critically ill haematological patients with ARF and respiratory virus detection. Hence, we retrospectively assessed prospectively collected CT scan, clinical and virology data. We hypothesized that: i) CT scan patterns will help to differentiate virus-only pneumonia from co-infections, ii) among virus-only infection patients, particular CT scan features will be associated to specific viruses, iii) CT scan data will have a predictive value for invasive mechanical ventilation (MV) and ICU mortality.
Methods
Study design and patient selection
This is retrospective observational study performed in ten University affiliated centers in France, belonging to a research network on critical respiratory diseases in patients with malignancies (“Groupe de Recherche en Réanimation Respiratoire en Onco-Hématologie”, GRRR-OH). All consecutive patients were included between January 1st, 2008 and April 8th, 2018. This study was approved by the Ethic Committee of University Teaching Hospital of Toulouse. Patient’s inclusion criteria were: adult ≥ 18 years of age, haematological malignancy (HM), ARF (PaO2 < 60mmHg or SpO2 < 90% ambient air, oxygen therapy ≥ 3 L/min for SpO2 > 92%, requiring more than 6 L/min, dyspnea or tachypnea > 30 cycles per minute at rest or cyanosis and signs of acute respiratory distress) [16], detection of one or more respiratory viruses by multiplex PCR from upper (nasopharyngeal swab or sputum) or lower (broncho-alveolar lavage) respiratory sample (Supplementary material1) [17-18]. Were excluded patients admitted to ICU for other reasons than ARF, and patients for whom CT scans were acquired more than 5 days before or after ICU admission.
Data collected through the medical record included baseline characteristics at ICU admission, microbiological sampling results, ICU evolution, final diagnosis of ARF and patient outcome. Comorbidities were assessed through Charlson index [19] and performance status through Eastern Cooperative Oncology Group (ECOG) score [20]. Disease severity and predicted mortality were quantified using Simplified Acute Physiology Score (SAPS) 2 and Sequential Organ Failure Assessment (SOFA) scores [21-22], while Kidney Disease Improving Global Outcome (KDIGO) classification [23] was used to determine and score acute renal injury.
Radiological procedures and CT scan criteria
Thoracic CT scan images were acquired in all patients. All CT scan were obtained in the supine position. Images were reconstructed with a slice thickness of 1 mm and an interval of 1 mm, respectively. Reconstructed images were transmitted to the workstation and picture archiving and communication systems (PACS) of Toulouse University Hospital. Images were analyzed by two radiologists (AN and MJ, both senior thoracic radiologists). All Digital Imaging and Communication in Medicine (DICOM) images from the CT studies were analyzed without access to clinical or laboratory findings. The evaluators independently and freely assessed the CT features using both axial CT images and multiplanar reconstruction images. After separate evaluations, any disagreement was resolved by discussion and consensus.
CT scan findings were preferentially defined according to the criteria of the Fleischner Society glossary of terms [24]. Bronchial radiological semiotics encompassed bronchial wall thickening, tree-in bud opacities, centro-lobular nodules and bronchial tubes dilatation. Interstitial syndrome was defined by the presence of lung reticulation, septal thickening and ground-glass opacities. Finally, alveolar damage was defined by the recognition of multifocal consolidations (Figure 2). An “aggregate lesion intensity score” was used to assess the total amount of lung parenchyma involvement and was defined by summing up individual score from 20 lung segments or 6 lung lobes (upper left lobe subdivided into culmen and lingula). Score of 0 or 1 were respectively assigned for each segment / lobe if an elementary lesion was detected.
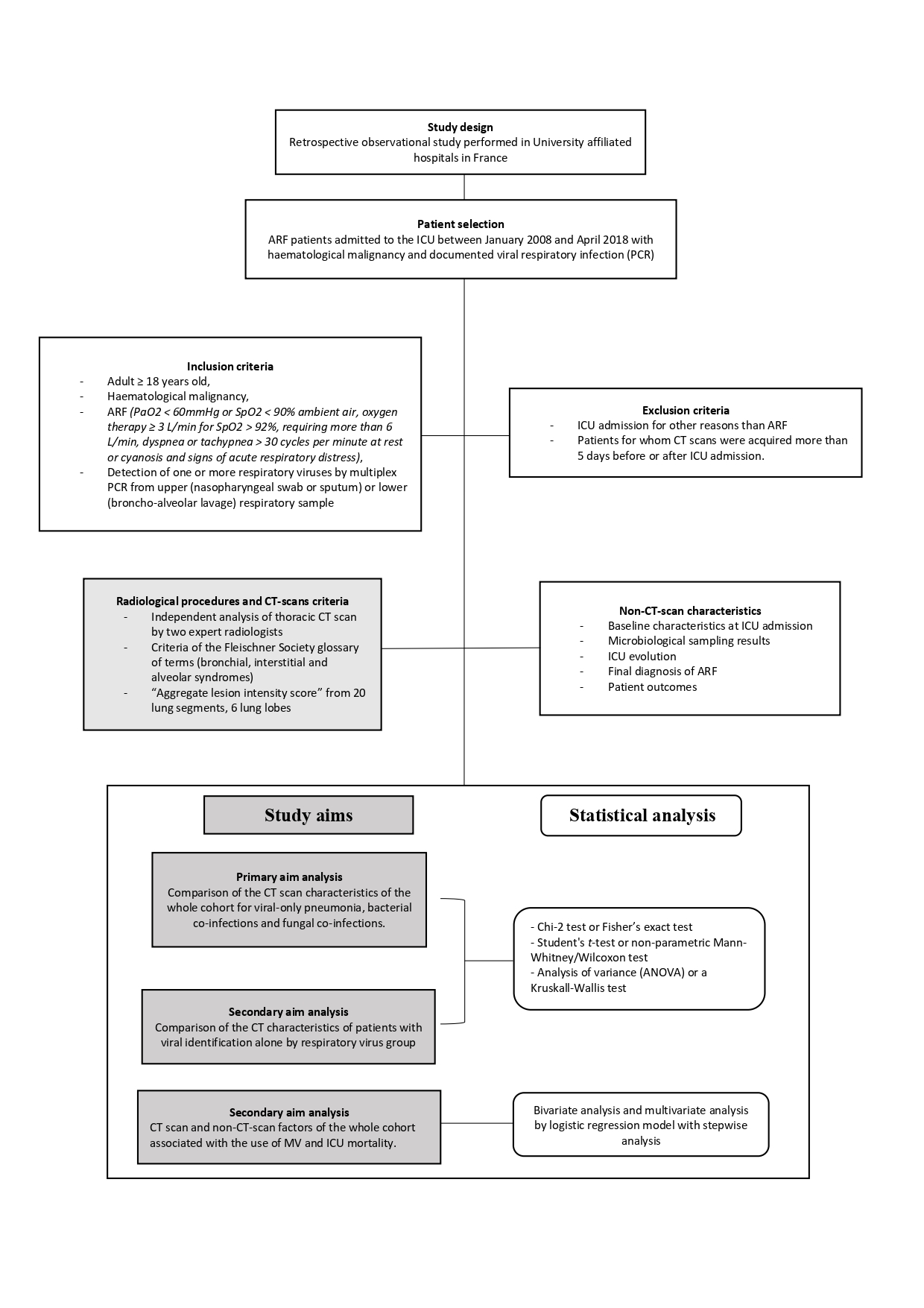
Figure 2:Methodology flowchart. ARF: acute respiratory failure, CT: computed tomography; ICU: intensive care unit; MV: mechanical ventilation; PCR: polymerase chain reaction.
Populations
The primary aim analysis was performed on the whole population, comparing CT data for viral-only pneumonia, bacterial co-infections and fungal co-infections. Then, the subpopulation which exclusively consisted of patients diagnosed with viral-only pneumonia was analysed. A comparison of the CT data was only performed for the groups of viruses which were represented in large enough numbers. Factors associated with the use of MV and ICU mortality, were analysed on the entire study population.
Statistical analysis
Statistical analyses were performed with the Stata software (Statistical Software: Release 14.2. Stata Corporation, College Station, Texas, USA). All reported p-values were two-sided and the significance threshold was set <5%. Quantitative data were described with their median and interquartile range (IQR). Qualitative data were described using number and frequency. Comparisons between groups were performed using either Chi-2 test or Fisher’s exact test, Student's t-test or non-parametric Mann-Whitney/Wilcoxon test, analysis of variance (ANOVA) or a Kruskall-Wallis test depending on the type of comparison being analysed
.The multivariate analysis was performed using a logistic regression model, including first non-collinear predictive variables (CT and non-CT characteristics) with a p-value lower than 0.20 in bivariate analysis. Stepwise analysis was then used to assess variables that were significantly and independently associated with the endpoints. Interactions between all potential confounding factors were tested and none were significant. Area under receiver operating characteristic (ROC) curve (AUC) was calculated to evaluate the discriminatory ability of each prediction model.
Results
Population
Table 1:Patients’ characteristics at ICU admission
| Number (%) or median [IQR] | Study population N=104 |
|---|---|
| Baseline characteristics | |
| Male gender | 66 (63.5) |
| Age (years) | 61 [53.5; 70.5] |
| Charlson comorbidity index | 4 [3; 5] |
| ECOG performance status ≤ 2 | 85 (81.7) |
| Hematological malignancy | |
| Lymphoid | 65 (62.5) |
| B/T cell lymphoma | 27 (26) |
| Multiple myeloma | 23 (22.1) |
| Chronic lymphocytic leukemia | 12 (11.5) |
| B/T-cell acute lymphoblastic leukemia | 3 (2.9) |
| Myeloid | 39 (37.5) |
| Acute myeloid leukemia | 27 (26.0) |
| Myelodysplastic / myeloproliferative disease | 12 (11.5) |
| Treatment phase | |
| Diagnosis/1st line | 54 (51.9) |
| ≥ 2nd line | 50 (48.1) |
| Malignancy status | |
| Newly diagnosed | 11 (10.6) |
| Complete or partial remission | 62 (59.6) |
| Progression | 13 (12.5) |
| Unknown | 18 (17.3) |
| Treatment in the previous month | 56 (53.8) |
| Time from haematological diagnosis to ICU admission (months) | 19.9 [3.5; 61.4] |
| Corticosteroids in the previous month | 58 (55.8) |
| Allogeneic stem cell transplantation | 23 (22.1) |
| Autologous stem cell transplantation | 23 (22.1) |
| Clinical symptoms | |
| Fever | 74 (71.2) |
| ENT symptoms | 11 (10.6) |
| Respiratory symptoms | 104 (100.0) |
| Dyspnea | 101 (97.1) |
| Cough | 51 (49.0) |
| Purulent sputum | 15 (14.4) |
| Focal or diffuse crackles | 24 (23.1) / 44 (42.3) |
| Wheezing | 28 (27.0) |
| Rhonchi | 22 (21.1) |
| Chest pain | 8 (7.7) |
| Laboratory parameters | |
| Lymphocyte count < 0.5 G/L | 64 (61.5) |
| Neutrophil count < 0.5 G/L | 36 (34.6) |
| Platelet count < 50 G/L | 47 (45.2) |
| Disseminated intravascular coagulation | 5 (4.8) |
| Lactic acidosis > 1 N | 25 (24.0) |
| Hypoalbuminemia (< 35 g/L), n = 67* | 67 (93.0) |
| KDIGO classification | |
| No acute renal failure / Stage 1 | 84 (80.8) |
| Stage 2 / Stage 3 | 20 (19.2) |
| Organ dysfunction scores | |
| SAPS II score | 56 [44; 67] |
| SOFA score day 1 | 8 [5; 11] |
ECOG : Eastern Cooperative Oncology Group; ENT : ear, nose and throat; ICU : intensive care unit; IQR : Inter Quartile Range (25th and 75th quartiles); KDIGO : Kidney Disease Improving Global Outcomes. *Missing data for 32 patients.
A total of 104 patients were enrolled during the study period (Figure 1). Patient characteristics at admission to ICU are described in table 1. The most frequently HM was acute myeloid leukaemia (26%), 54% of cohort patients were at diagnosis or in the first-line therapy, 59.6% were in remission and 22.1% had received an allo-SCT. Median SAPS 2 and SOFA scores were 56 [IQR: 44; 67] and 8 [5; 11] respectively. All patients had respiratory symptoms (dyspnea: 97.1%, crackles: 65.4%, cough: 49% and wheezing: 27%) whereas only 10.6% had ear nose and throat (ENT) symptoms and 71.2% of them were febrile. Severe neutropenia and lymphocytopenia were observed in 36 (35.3%) and 64 (62.7%) of patients, respectively.
Concerning life-supporting interventions (Table 2), high-flow oxygen or non-invasive ventilation was initiated in 42.3% and 48.1% of patients. MV (invasive mechanical ventilation) was required in 67.3% (n = 70) of patients after a median waiting time of 0 [0; 1] days and for a median length of 9 [5; 19] days. Over half of the patients (57/104) had ARDS (54.8%). Five patients (4.8%) required veno-venous extra corporeal membrane oxygenation (VV-ECMO) assistance. Catecholamines and renal replacement therapy (RRT) were initiated in 67 (64.4%) and 18 (17.0%) of patients, respectively. Multiple organ dysfunction syndrome (MODS) was observed in 37 patients (35.6%). The median duration of the ICU stay was 9 [5; 17] days. ICU and hospital mortality were 40.4% and 46.2%, respectively.
Microbiological diagnosis
Regarding the diagnosis of respiratory viruses, proximal alone, distal alone and both proximal and distal samples were positive in 33.7% (n=35/104), 42.3% (n=44/104) and 24.0% (n=25/104) respectively (Supplementary Table 1). 93.2% (n = 69/74) of distal samples and 91.0% (n = 60/66) of proximal samples were positive. Forty-three (41.3%) airway samples were positive for Influenza, 23 (22.1%) for respiratory syncytial virus (RSV), 15 (14.4%) for human Metapneumovirus (hMPV), 14 (13.5%) for Rhinovirus/Enterovirus, 10 (9.6%) for Parainfluenza virus (PIV), 9 (8.7%) for Coronavirus, and 1(1%) for Bocavirus. In 10.6% (n = 11) of cases the same patient was infected with two viruses (Supplementary Table 1). Thirty-nine (37.5%) patients received oseltamivir and 13 (12.5%) ribavirin. Sixty (57.7%) patients had virus-only infections (4 patients had a non-respiratory viral co-infection), 17 (16.3%) patients had an isolated bacterial co-infection, 10 (9.6%) an isolated Pneumocystis jirovecii co-infection, 8 (7.7%) an isolated fungal co-infection and 9 (8.7%) patients had multiple co-infections (Figure 1, Supplementary table 2). Almost all (n = 103, 99%) of the cohort initially received anti-bacterial treatments, while 60.6% of patients (n = 63) anti-fungal treatments and only 16.4% (n = 17) non-respiratory anti-viral treatments.
Table 2:Patient’s characteristics during ICU follow up.
| Number (%) or median [IQR] | Study population N=104 |
|---|---|
| Study population | |
| SOFA score day 7 | 8 [4; 14] (n= 71) |
| Delta SOFA (SOFA day 7 - SOFA day 1) | 0 [-3; 5] (n= 71) |
| Life-supporting interventions | |
| Standard oxygen therapy | 10 (9.6) |
| High-flow oxygen therapy | 44 (42.3) |
| Non-invasive mechanical ventilation | 50 (48.1) |
| Invasive mechanical ventilation | 70 (67.3) |
| Invasive mechanical ventilation - delay since ICU admission (days) | 0 [0; 1] |
| Invasive mechanical ventilation - length (days) | 9 [5; 19] |
| ARDS | 57 (54.8) |
| ECMO | 5 (4.8) |
| Catecholamines | 67 (64.4) |
| Renal replacement therapy | 26 (25) |
| MODS | 37 (35.6) |
| Final ARF aetiological diagnosis | |
| Infectious pneumonia | 104 (100) |
| Viral-only | 60 (57.7) |
| Bacterial co-infection | 17 (16.3) |
| Pneumocystis jirovecii co-infection | 10 (9.6) |
| Filamentous fungal co-infection | 8 (7.7) |
| Multiple co-infections | 9 (8.7) |
| Acute pulmonary cardiogenic oedema | 5 (4.8) |
| Pulmonary embolism | 4 (3.9) |
| Pleural effusion | 2 (1.9) |
| Malignancy-related pulmonary involvement | 1 (1) |
| Outcomes | |
| Limitation or discontinuation of invasive treatments | 31(29.8) / 17 (16.3) |
| ICU LOS (days) | 9 [5; 17] (n=104) |
| Hospitalisation LOS (days) | 26 [14; 47] (n=102) |
| ICU mortality | 42 (40.4) |
| Hospital mortality | 48 (46.2) |
| Overall survival since ICU admission [n=66/104] (months) | 3.4 [0.4; NR] |
ARDS: acute respiratory distress syndrome; ARF: acute respiratory failure; ECMO: Extracorporeal Membrane Oxygenation; ICU: intensive care unit; IQR: interquartile range (25th and 75th quartiles); LOS: length of stay; MODS: multiple organ dysfunction syndrome; NR: not reached; SAPS II: Simplified Acute Physiology Score II; SOFA: Sequential Organ Failure Assessment.
Analysis according radiological data
As regards iconographic data, thoracic radiography was anomalous in 94.1% (95/101) of cases (diffuse interstitial (55.8%), diffuse alveolar (41.3%) or focal (25%) abnormalities). All patients underwent a thoracic CT scan, which was abnormal in all cases. Intravenous contrast agents were used in 54.8% of CT scans.
CT scan characteristics of the whole cohort.
CT scan characteristics of the whole cohort (n = 104) are summarized in Figure 2. Alveolar syndrome was most frequently encountered (n = 72, 69.2%) followed by bronchial syndrome (n = 61, 58.7%) and interstitial syndrome (n = 59, 56.7%). The most prevalent primary lesions were centro-lobular nodules (n = 56, 53.8%) and tree-in-bud opacities (n = 35, 33.7%) for bronchial syndrome and ground-glass opacification (n = 52, 50.0%) for interstitial changes. The median aggregate lesion intensity extended to 12 [8.0; 17.3] segments and 5 [3.0; 5.0] lobes.
To assess the added value of thoracic CT scan to disentangle virus-only from respiratory co-infections, we compared patients with respiratory virus alone, patients with viral and bacterial co-infection and finally, patients with viral infection plus multiple co-infections, including fungal infections (Supplementary table 3). We only observed a significant difference in the case of interstitial lesions which were more frequently associated with a virus-only infection (n = 36, 60%) or with a fungal co-infection (n = 18, 66.7%) than with a bacterial co-infection (n = 5, 29.4%) (p = 0.038). No significant differences were detected for bronchial, alveolar syndromes or in terms of lesion intensities.
Table 3:Multivariate analysis of factors independently associated with invasive mechanical ventilation and ICU mortality. Aggregate lesion intensity score/number of lobes is a quantitative variable so OR corresponds to an increase for a lobe.
| Associated factors | Adjusted odds ratio | 95% CI | P value |
|---|---|---|---|
| INVASIVE MECHANICAL VENTILATION | |||
| Bronchial syndrome | 0.21 | [0.06; 0.69] | 0.01 |
| Aggregate lesion intensity score/number of lobes | 1.88 | [1.27; 2.80] | 0.002 |
| SAPS II score | |||
| < 56 (median) | 1 | < 0.001 | |
| ≥ 56 | 20.72 | [5.45; 78.79] | |
| AUC | 0.88 [0.82; 0.94] | ||
| ICU MORTALITY | |||
| Aggregate lesion intensity score/number of lobes | 1.64 | [1.10; 2.44] | 0.015 |
| SAPS II score | |||
| < 56 (median) | 1 | 0.009 | |
| ≥ 56 | 4.37 | [1.45; 13.17] | |
| Lactic acidosis | 5.82 | [1.55; 21.80] | 0.009 |
| Multiple organ dysfunction syndrome | 12.04 | [3.76; 38.56] | <0.001 |
| AUC | 0.90 [0.84; 0.96] |
AUC: area under ROC curve (Receiver Operating Characteristic); 95% CI: 95% confidence interval; ICU: intensive care unit; OR: odds-ratio; SAPS II: Simplified Acute Physiology Score
CT scan characteristics of patients with viral identification alone
Regarding the ability of radiological appearances to diagnose specific virus species among virus-only infection, we analysed CT scan features of 56 patients (53.8%) after excluding patients with associated parenchymal disease (Figure 1). Analysis of CT scan characteristics by respiratory virus group was performed by comparing the two main virus groups of our cohort: Orthomyxovirus alone (37.5% of patients with Influenza) and Paramyxovirus/Pneumovirus alone (46.4% of patients with RSV or hMPV or PIV) (Supplementary table 4). The most frequently observed primary lesions were centro-lobular nodules (n = 29, 51.8%) and tree-in-bud opacities (n = 20, 35.7%) for bronchial syndrome (n = 33, 58.9%); ground-glass opacification (n = 29, 51.8%) for interstitial changes (n = 33, 58.9%) and consolidations (n = 35, 62.5%) for alveolar syndrome. The most prevalent associations occurred with alveolo-interstitial (39.3%) and broncho-alveolar (33.9%) syndromes. The median aggregate lesion intensity extended to 12 [8.0; 18.0] segments and 5 [3.0; 5.0] lobes. Alveolar syndrome was notably, but not significantly, overrepresented in patients with Orthomyxovirus infections (76.2% vs 50%, p=0.066). This was also the case for broncho-alveolar syndrome, but the association reached statistical significance in this particular instance (52.4% vs 15.4%, p=0.007). The median aggregate lesion expressed in number of segments was higher for Orthomyxovirus (16 [8; 20] vs 11.5 [6; 15]) but did not reach statistical significance (p = 0.067).
Outcome assessments
Finally, we have assessed the potential relationship between CT scan data and patient’s outcome, in term of need of MV or ICU mortality. CT scan patterns and aggregate lesion intensity scores were significantly associated with MV requirement or ICU mortality (Table 3, Supplementary table 5,6 and Supplementary figure1). In multivariate analysis, aggregate lesion intensity score (number of lobes) was significantly and independently associated with MV and ICU mortality (OR =1.88 for each lobe with lesion [95% CI: 1.27; 2.80], p = 0.002 and OR =1.64 [95% CI: 1.10; 2.44], p = 0.002; respectively) with a cumulative effect based on the number of affected lobes. Whereas, bronchial syndrome was significantly inversely associated with MV (OR =0.21 [95% CI: 0.06; 0.69], p = 0.010. For non-CT scan characteristics, SAPS II score (≥ median) was associated with MV (OR =20.72 [95% CI: 5.45; 78.79], p < 0.001) and SAPS II score (≥ median), lactic acidosis and MODS were associated with ICU mortality (OR =4.37 [95% CI: 1.45; 13.17], p = 0.009; OR =5.82 [95% CI: 1.55; 21.80], p = 0.009; OR =12.04 [95% CI: 3.76; 38.56], p < 0.001 respectively).
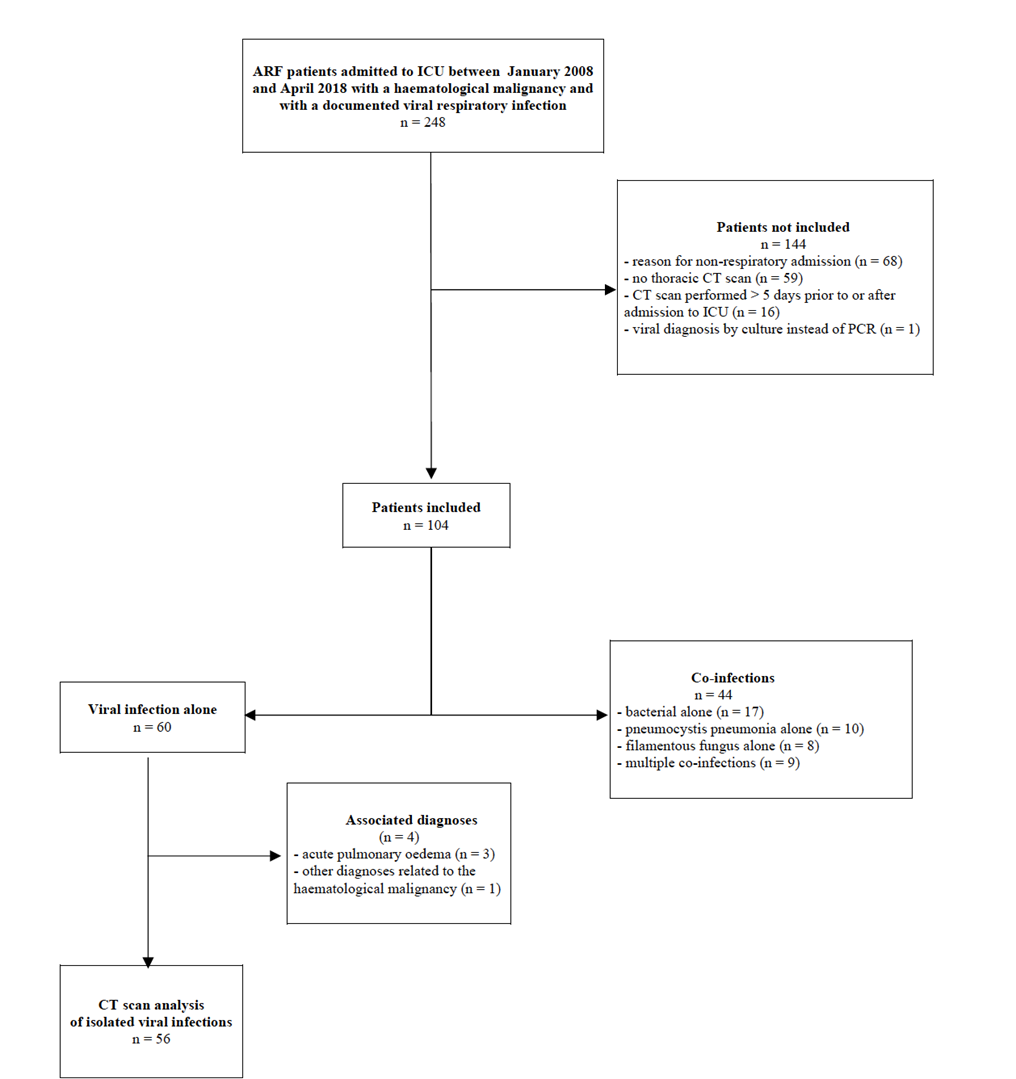
Figure 1:Study Flowchart. ARF: acute respiratory failure; CT scan: computed tomography scan; ICU: intensive care unit.
Discussion
Among the different infectious agents which cause pulmonary infection in critically ill haematological patients, pneumonia caused by respiratory virus has been associated with a particularly high risk of death [3-5]. Although there is no recommendation for routine use of radiological assessments in this challenging clinical setting [3, 25], chest CT scan is broadly used. The vast majority of reported studies in this field, have almost exclusively addressed the description of radiological semiotics [25-29], but did not provide the still lacking information about the usefulness of chest CT scan in terms of diagnosis and prognostication in this population. To the extent of our knowledge, our study provides the first evidence about the added clinical value of early performed chest CT scan in critically ill immunocompromised patients with ARF and respiratory viral detection, both to facilitate the etiological diagnosis and to predict patient’s ICU clinical course and outcome.
The radiological appearances of viral lower respiratory tract infection in immunocompromised patients are unclear, because they are frequently accompanied by infections by bacteria, fungi or other virus, and data on the radiologic findings of the individual viruses are very limited [30-34]. Through a careful comparison between CT-scan data from patients with virus-only and bacterial/fungal co-infection we have clearly identified that a radiological interstitial pattern was more frequently observed in patients with viral infection alone or with fungal co-infection. Opposite to previous reports, alveolar pattern detection, corresponding to a significant lung loss of aeration and generally associated to the diagnosis of bacterial pneumonia in immunocompetent patients, was not solely related to bacterial co-infection in our cohort. We think that our data support the idea of combining radiological examination and adapted microbiological examination to accurately identify potential overlapping co-infection.
Aiming to assess the ability of CT scan data to diagnose specific virus species among virus-only infection, we analysed pre-defined specific radiological features after excluding patients with associated parenchymal disease. In line with previous reports [35-37], bronchial, interstitial and alveolar radiological patterns were evenly distributed across all respiratory virus groups. Interestingly, we have identified a higher prevalence of a broncho-alveolar association in the Orthomyxovirus (52.4%) group when compared to the Paramyxovirus/Pneumovirus group (15.4%). We suggest that this association may provide supportive evidence in favour of an early empirical treatment with a neuraminidase inhibitor in this specific case [38-42].
Besides the impact in terms of diagnosis, this study identifies two radiological factors significantly and independently associated with the use of invasive mechanical ventilation and patient’s ICU mortality, namely the identification of a bronchial features and the aggregate lesion intensity score. Overall, the bronchial radiological pattern was associated to better patient’s ICU outcome. We think that this result is in agreement with the current view of acute respiratory distress syndrome pathophysiology [43], which posits that a bronchial, rather that alveolar damage, should have a lesser impact on alveolar gas exchange, and therefore in terms of ARF severity and potential of recovery. And last but not the least, we also identified a significant relationship between the total amount of lung injury rated by the aggregate lesion integrity score and patient’s outcome. Future prospective studies are warranted to further assess and potentially validate this promising radiological tool for prognostication.
Our results must be interpreted with caution and a number of limitations should be borne in mind. The first is related to the study retrospective design, and a lengthy study inclusion period, extended back to 2008 and prolonged over a ten-year period. Therefore, we cannot eliminate changes in thoracic imaging procedures, PCR kits performance or clinical care across this period. Hence, a prospective validation of these findings is certainly needed. As a second limitation, we acknowledge that the limited sample size limits the generalizability of our finding and might be responsible of the reported modest p-value associations and broad calculated 95% confidence intervals for Odds Ratio. Consequently, the reported evidence requires confirmation from large-scale perspective trials with strict recruitment criteria. The main strengths of our study are the multicentric inclusions with pre-defined, centralized and independent evaluation of the radiological findings. Actually, ours is probably the largest detailed description of CT scan findings in critically ill haematological patients with acute respiratory failure and respiratory virus detection.
In conclusion, we observed that: i) the broncho-alveolar radiological feature was more frequently observed in patients infected by Orthomyxovirus, ii) the presence of bronchial radiological pattern and the extension of lung injuries were significantly related to patient’s outcome. Future studies should focus on prospectively testing the prognostic value of these radiological markers. The poor outcome that we report, highlight the need of further research to design more personalized intervention strategies for these patients, which will be at least in part build upon CT scan data.
Author Contributions
Dr. Silva had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Picard, Amazigh, Bérard, Silva. Acquisition of data: Picard, Amazigh, Bouharaoua, Mansuy, Mengelle, Morel, Cadoz, Platon, Ferre, Verlhac, Issa, Argaud, Geri, Reuter, Ducos, Ramonda, Ruiz. Analysis and interpretation of data: Fernandez, Bérard, Picard, Amazigh, Silva. Drafting of the manuscript: Picard, Amazigh, Bérard, Silva. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Fernandez, Bérard. Administrative, technical, or material support: Récher, Argaud, Azoulay. Study supervision: Silva.
Conflicts of Interest
The authors have no conflict of interest to disclose.
References
1. Azoulay E, Mokart D, Pène F, et al. Outcomes of Critically Ill Patients with Hematologic Malignancies: Prospective Multicenter Data from France and Belgium—A Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique Study. JCO. 2013; 31:2810–2818.
2.For the Efraim investigators and the Nine-I study group, Azoulay E, Pickkers P, et al. Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med. 2017; 43:1808–1819.
3. the Nine-i Investigators, Azoulay E, Russell L, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 2020; 46:298–314.
4.Contejean A, Lemiale V, Resche-Rigon M, et al. Increased mortality in hematological malignancy patients with acute respiratory failure from undetermined etiology: a Groupe de Recherche en Réanimation Respiratoire en Onco-Hématologie (Grrr-OH) study. Ann Intensive Care. 2016; 6:102.
5. Legoff J, Zucman N, Lemiale V, et al. Clinical Significance of Upper Airway Virus Detection in Critically Ill Hematology Patients. Am J Respir Crit Care Med. 2019; 199:518–528.
6. Schnell D, Mayaux J, Lambert J, et al. Clinical assessment for identifying causes of acute respiratory failure in cancer patients. Eur Respir J. 2013; 42:435–443.
7. Tiveljung-Lindell A, Rotzén-Östlund M, Gupta S, et al. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses: High Throughput Diagnostics. J Med Virol. 2009; 81:167–175.
8. Sanghavi SK, Bullotta A, Husain S, et al. Clinical evaluation of multiplex real-time PCR panels for rapid detection of respiratory viral infections. J Med Virol. 2012; 84:162–169.
9. Wohlfarth P, Turki AT, Steinmann J, et al. Microbiologic Diagnostic Workup of Acute Respiratory Failure with Pulmonary Infiltrates after Allogeneic Hematopoietic Stem Cell Transplantation: Findings in the Era of Molecular- and Biomarker-Based Assays. Biology of Blood and Marrow Transplantation. 2018; 24:1707–1714.
10. Lachant DJ, Croft DP, McGrane Minton H, et al. Nasopharyngeal viral PCR in immunosuppressed patients and its association with virus detection in bronchoalveolar lavage by PCR: Association of NP and BAL viral PCR. Respirol. 2017; 22:1205–1211.
11. von Lilienfeld-Toal M, Berger A, Christopeit M, et al. Community acquired respiratory virus infections in cancer patients—Guideline on diagnosis and management by the Infectious Diseases Working Party of the German Society for haematology and Medical Oncology. Europ J Canc. 2016; 67:200–212.
12. Cortez KJ, Erdman DD, Peret TCT, et al. Outbreak of Human Parainfluenza Virus 3 Infections in a Hematopoietic Stem Cell Transplant Population. J Infect Dis. 2001; 184:1093–1097.
13. Miller WT, Mickus TJ, Barbosa E, et al. CT of Viral Lower Respiratory Tract Infections in Adults: Comparison Among Viral Organisms and Between Viral and Bacterial Infections. Am J Roentgenol. 2011; 197:1088–1095.
14. Koo HJ, Lim S, Choe J, et al. Radiographic and CT Features of Viral Pneumonia. RadioGraphics. 2018; 38:719–739.
15. For the Efraim investigators and the Nine-I study group, Martin-Loeches I, Lemiale V, et al. Influenza and associated co-infections in critically ill immunosuppressed patients. Crit Care. 2019; 23:152.
16. Azoulay É, Mokart D, Lambert J, et al. Diagnostic Strategy for Hematology and Oncology Patients with Acute Respiratory Failure: Randomized Controlled Trial. Am J Respir Crit Care Med. 2010; 182:1038–1046.
17. Mahony JB. Detection of Respiratory Viruses by Molecular Methods. Clin Microbiol Rev. 2008; 21:716–747.
18. Afonso CL, Amarasinghe GK, Bányai K, et al. Taxonomy of the order Mononegavirales: update 2016. Arch Virol. 2016; 161:2351–2360.
19. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987; 40:373–383.
20. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655
21. Le Gall JR. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA: The J Am Med Assoc. 1993; 270:2957–2963.
22. Vincent J-L, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine (see contributors to the project in the appendix). Intensive Care Med. 1996; 22:707–710. h
23. The ad-hoc working group of ERBP, Fliser D, Laville M, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines on Acute Kidney Injury: Part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dialysis Transplantation. 2012; 27:4263–4272.
24. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiol. 2008; 246:697–722.
25. Azoulay E, Mokart D, Kouatchet A, et al. Acute respiratory failure in immunocompromised adults. The Lancet Resp Med. 2019; 7:173–186.
26. Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012; 119:2738–2745. https://doi.org/10.1182/blood-2011-08-371112
27. Kim Y-J, Guthrie KA, Waghmare A, et al. Respiratory Syncytial Virus in Hematopoietic Cell Transplant Recipients: Factors Determining Progression to Lower Respiratory Tract Disease. The J Infect Dis. 2014; 209:1195–1204.
28. Vakil E, Evans SE. Viral Pneumonia in Patients with Hematologic Malignancy or Hematopoietic Stem Cell Transplantation. Clin Chest Med. 2017; 38:97–111.
29. Kunihiro Y, Tanaka N, Kawano R, et al. Differential diagnosis of pulmonary infections in immunocompromised patients using high-resolution computed tomography. Eur Radiol. 2019; 29:6089–6099.
30. Gasparetto EL, Escuissato DL, Marchiori E, et al. High-Resolution CT Findings of Respiratory Syncytial Virus Pneumonia After Bone Marrow Transplantation. Am J Roentgenol. 2004; 182:1133–1137.
31. Mayer J, Lehners N, Egerer G, et al. CT-Morphological Characterization of Respiratory Syncytial Virus (RSV) Pneumonia in Immune-Compromised Adults. Fortschr Röntgenstr. 2014; 186:686–692.
32. Koo HJ, Lee HN, Choi SH, et al. Clinical and Radiologic Characteristics of Human Metapneumovirus Infections in Adults, South Korea. Emerg Infect Dis. 2019; 25:15–24.
33. Ferguson PE, Sorrell TC, Bradstock KF, et al. Parainfluenza Virus Type 3 Pneumonia in Bone Marrow Transplant Recipients: Multiple Small Nodules in High‐Resolution Lung Computed Tomography Scans Provide a Radiological Clue to Diagnosis. Clin Infect Dis. 2009; 48:905–909.
34. Franquet T, Rodríguez S, Martino R, et al. Human Metapneumovirus Infection in Hematopoietic Stem Cell Transplant Recipients: High-Resolution Computed Tomography Findings. J Computer Assisted Tomography. 2005; 29:223–227.
35. Shiley KT, Van Deerlin VM, Miller WT. Chest CT Features of Community-acquired Respiratory Viral Infections in Adult Inpatients With Lower Respiratory Tract Infections. J Thoracic Imaging. 2010; 25:68–75.
36. Kim M-C, Kim MY, Lee HJ, et al. CT findings in viral lower respiratory tract infections caused by parainfluenza virus, influenza virus and respiratory syncytial virus. Med. 2016; 95:e4003.
37. Franquet T, Rodriguez S, Martino R, et al. Thin-Section CT Findings in Hematopoietic Stem Cell Transplantation Recipients with Respiratory Virus Pneumonia. Am J Roentgenol. 2006; 187:1085–1090.
38. On behalf the “Flu in Lyon ICUs” Study Group, Hernu R, Chroboczek T, et al. Early oseltamivir therapy improves the outcome in critically ill patients with influenza: a propensity analysis. Intensive Care Med. 2018; 44:257–260.
39. Lytras T, Mouratidou E, Andreopoulou A, et al. Effect of Early Oseltamivir Treatment on Mortality in Critically Ill Patients With Different Types of Influenza: A Multiseason Cohort Study. Clin Infect Dis. 2019; 69:1896–1902.
40. Waghmare A, Englund JA, Boeckh M. How I treat respiratory viral infections in the setting of intensive chemotherapy or hematopoietic cell transplantation. Blood. 2016; 127:2682–2692.
41. Chemaly RF, Shah DP, Boeckh MJ. Management of Respiratory Viral Infections in Hematopoietic Cell Transplant Recipients and Patients With Hematologic Malignancies. Clin Infect Dis. 2014; 59:S344–S351.
42. Hirsch HH, Martino R, Ward KN, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): Guidelines for Diagnosis and Treatment of Human Respiratory Syncytial Virus, Parainfluenza Virus, Metapneumovirus, Rhinovirus, and Coronavirus. Clin Infect Dis. 2013; 56:258–266
43. West JB, Luks A. West’s respiratory physiology: the essentials, Tenth edition. Wolters Kluwer, Philadelphia
Received: August 08, 2022;
Accepted: September 19, 2022;
Published: September 22, 2022.
To cite this article : Picard M, Aguersif A, Bérard E, et al. Usefulness of Chest CT Scan in Haematological Patients with Severe Acute Respiratory Failure and Respiratory Virus Detection. European Journal of Respiratory Medicine. 2022; 4(2): 334 - 343. doi: 10.31488/EJRM.135.
© 2022 Picard M, et al.
