Review Article/ Open Access
DOI:10.31488/EJRM.130
Vaccine Delivery by Precipitation (VDBP) Lessons from Poison Ivy for Protein Antigens in General and Specifically for SARS Co-V2
Robert E. Coifman M.D1, Catherine F. Yang Ph.D2
1. Allergy & Asthma of South Jersey, P. A, Millville NJ, USA
2. College of Medicine, California Northstate University, Elk Grove, CA
*Corresponding author: Robert E. Coifman, Allergy & Asthma of South Jersey, P. A, Millville NJ, USA
Abstract
Vaccine Delivery by Precipitation (VDBP) is the precipitation of hundreds of thousands to millions of micron-sized particles of a water-insoluble antigen within a volume of a recipient tissue as a water-miscible solvent in which the antigen was administered is diluted by the water content of the recipient tissue. Particles sized 0.5 to 5 microns, taken up by dendritic cells by “macropinocytosis,“ are presented to naive T cells for immunomodulation with sufficient potency to have achieved the world’s first induction of tolerance in patients previously sensitized to poison ivy. Here, we discuss VDBP applications to therapeutic modulation of immunologic reactivity to proteins. These include immunomodulation from sensitization to tolerance in allergic diseases. They also include immunomodulation from tolerance ro protective sensitization in cancer and from immunological naivete (lack of any current response because of lack of prior exposure) to protective sensitization for infectious diseases. We discuss the development of a VDBP vaccine for SARS Cov-V2 as a specific application of the latter technology.
Introduction
Poison ivy (PI) east of the Continental Divide and poison oak (PO) in the drier climate of the West are the members of the genus Toxicodendron most commonly associated with allergic contact dermatitis. They are endemic in all states of the US except Alaska and Hawaii, all provinces of Canada except Newfoundland, Nunavut and the Northwest Territories, and in the mountain ranges and highlands of Mexico. They produce urushiols, catechols with side chains of either 15 or 17 carbons with zero to three unsaturated bonds near their distal ends. Urushiols bond oxidatively to keratin and other skin proteins where they act as haptens. The Japanese lacquer tree is a member of the genus that migrated to Asia when there was a land bridge between Siberia and Alaska. Parts of other plants of the family Anacardiaceae including mango skin and cashew shells also contain allergenic urushiols.
Eighty five per cent of persons living in the U. S. will become sensitized to urushiols if sufficiently exposed. Fifty per cent will have symptoms requiring medical care at some point in their lifetime [1]. Isolated occurrences elsewhere are typically caused by growth from accidentally transported seeds.
Reports of unsuccessful and unconfirmed efforts to induce tolerance in already sensitized persons span more than 100 years [2, 3]. Previously licensed PI and PO allergy vaccines were removed from the US market in 1994 when a rule change required demonstration of efficacy in addition to demonstration of safety and no manufacturer of a previously licensed vaccine submitted efficacy data to the FDA.
The authors were impressed that those vaccines offered some benefit to some patients so in 2008 offered to make a vaccine from locally growing PI for a PI-allergic professional tree trimmer who required 40 mg per day for 10 months of every year to be able to work. Previously licensed vaccines were made from urushiols extracted from fresh or dried leaves with ether, evaporated to dryness and redissolved in sterile vegetable oils for either ingestion or subcutaneous (SQ) injection. Without an aseptic processing facility to maintain sterility in vegetable oils we adopted the expedient of extracting with ethanol, assaying for urushiol content, presuming the ethanol to be self-sterilizing and injecting small volumes of the same crude ethanol extract as our first generation vaccine. We gave one or more injections of up to 0.15 ml to the deltoid muscle, choosing the IM route instead of SQ so the ethanol, being a tissue irritant, would be more rapidly diluted by the greater available water content of muscle. To our surprise this patient and also a second of our first 4 patients achieved complete clinical tolerance with a total treatment dose of only 0.8 mg. Our index patient lost tolerance by 14 months but during that time fell from a tree and left that occupation. Adding urushiol concentrate to achieve a total treatment dose ~20 mg while limiting maximum injection volume to 0.15 ml increased efficacy to 90% with median duration of protection > 2 years. Our adverse effects profile per course of treatment was less than ordinary injection allergen immunotherapy. Of patients who lost tolerance and requested re-treatment or experienced a suboptimal response to initial treatment, 100% response to booster [4].
Interpretation
What did we accomplish that others failed to do for more than 100 years? We injected a water-insoluble antigen in a pharmaceutically acceptable water-miscible solvent that carried antigen with it as it spread from the injection site. As the small volume of injected ethanol was diluted by the water content of the recipient tissue the urushiol became insoluble and precipitated. The faster the rate of solvent dilution the larger the number and smaller the size of the resulting particles. Particles in the 0.5 to 5 micron size range are efficiently taken up by naive dendritic antigen-presenting cells (APCs’s) by macropinocytosis [5]. We had the good fortune to achieve a balance between injected volume, viscosity and access to tissue water such that the rate at which the injected ethanol was diluted by tissue water caused the dissolved uruishiol to loose solubility and precipitate at a rate yielding hundreds of thousands to millions of particles in the size range of APC uptake by macropinocytosis. We’d chosen a tissue (skeletal muscle) in which the primary evolutionary role of the immune system is the maintenance of tolerance to self. If our interpretation is correct we would expect the lineages of dendritic cell populations in muscle to be primarily tolerogenic. and the cytokine milieu of the lymph nodes to which those dendritic cells bring antigen to present to naive T cells to be similarly biased toward immunomodulation from sensitization to tolerance.
Current understanding of the cellular mechanisms of immunomodulation is illustrated by Figure 1, adapted from Teunissen [6]. The locus is the cortex of a lymph node draining the site of vaccine administration. Dendritic cells (DC’s) from either homeostatic or inflamed tissue present antigenic epitopes in their MHC Class II molecular grooves, to naive T lymphocytes (Tn). These can tolerize by maturing into Treg cells, sensitize by maturing along any of the other illustrated tracks, and immunomodulate by outnumbering and displacing already present T-cells of one type with those of another. The cellular and molecular mechanisms are the same for immunomodulation from sensitization to tolerance in allergy and from tolerance in cancer and naivete or tolerance in infectious diseases to protective sensitization. The switches are simply flipped in different directions [7]. In the terminology of physics, the system has inertia and it takes force to change its direction. Our VDBP vaccine successfully changed the direction of the immune response in patients already sensitized to poison ivy patients whereas 100 years of administration of the same antigen by other routes had failed. We propose that the amplification of immunomodulatory potency we observed by feeding antigen to dendritic cells by macropinocytosis is a general phenomenon and that VDBP can facilitate the flipping of other previously flip-resistant switches of immunological responsiveness, as well. The urushiols of PI and PO naturally have the solubility properties needed for VDBP: Insolubility in water combined with sufficient solubility in at least one of the three pharmaceutically acceptable water miscible solvents (ethanol, acetonitrile and dimethyl sulfoxide (DMSO)) for a treatment dose to be dissolved in a pharmaceutically acceptably small volume. In greater detail, the administered volume of solvent must be such that the rate at which it’s diluted by available tissue water causes the vaccine to lose solubility and precipitate into particles in the size range of 0.5 to 5 microns which are efficiently taken up by naïve dendritic APCs by macropinocytosis.
How important are vaccine physical from (solution or particulate) and particle size? Particulate form with particles in the 0.5 to 5 micron size range capable of dendritic cell uptake by macropinocytosis was proven superior to soluble forms of the same antigens for immunomodulation both from sensitization to tolerance [8] and from naiveté to sensitization [9].
Other similarly sized particulate vaccines are not VDBP:
Most nanoparticle and liposome manufacturing technologies can be adapted to make particles in the 0.5 to 5 micron size range. However, neither these nor any other pre-manufactured particulate vaccine can be administered by VDBP. In VDBP, particles are created de novo in situ, dispersed in a volume of a non-liquid recipient tissue. Every living non-liquid tissues is patrolled by naive (not having previously taken up antigen) dendritic APCs looking for gaps in homeostasis. We speculate that the irritant effect of the solvent from which the particles precipitate may be a signal that increases dendritic cell trafficking. There is no way that pre-formed nano/microparticle or liposomal vaccines can be dispersed within a volume of a recipient tissue with the dendritic cell exposure of VDBP.
Application to protein antigens in general:
Our challenge, to give vaccines for protein antigens the immunomodulatory potency of feeding to dendritic cells by macropinocytosis, is to find a way to give such vaccines the solubility properties needed for VDBP. We believe this can be accomplished with peptide vaccines containing the relevant epitopes, formulated to also be insoluble in water, and with treatment doses soluble in sufficiently small volumes of one or more of ethanol, acetonitrile and DMSO.
The concept of manufacturing overlapping peptide vaccines to treat allergies to protein allergens was developed as a strategy to induce immunomodulation from sensitization to tolerance without the vaccines themselves being able to trigger anaphylaxis. In Figure 1 this represents presentation by dendritic cells (DC) to naïve T lymphocytes (TN) of peptides containing epitopes capable of inducing immunomodulation from antibody production (Tfh in the figure) to tolerance (Treg) but in forms incapable of crosslinking molecules of antigen-specific IgE (shown graphically at the lower right corner of the figure) attached to mast cells. This is attempted with peptides containing known relevant or potentially relevant AA sequences up to the maximum 9 AA chain length capacity of the antigen presenting grooves of the recipient’s dendritic cell MHC class II molecules [10] but either physically too short or structurally lacking either homologous [11] or heterologous [12] bivalency that could cross-link IgE molecules on mast cells and trigger degranulation..
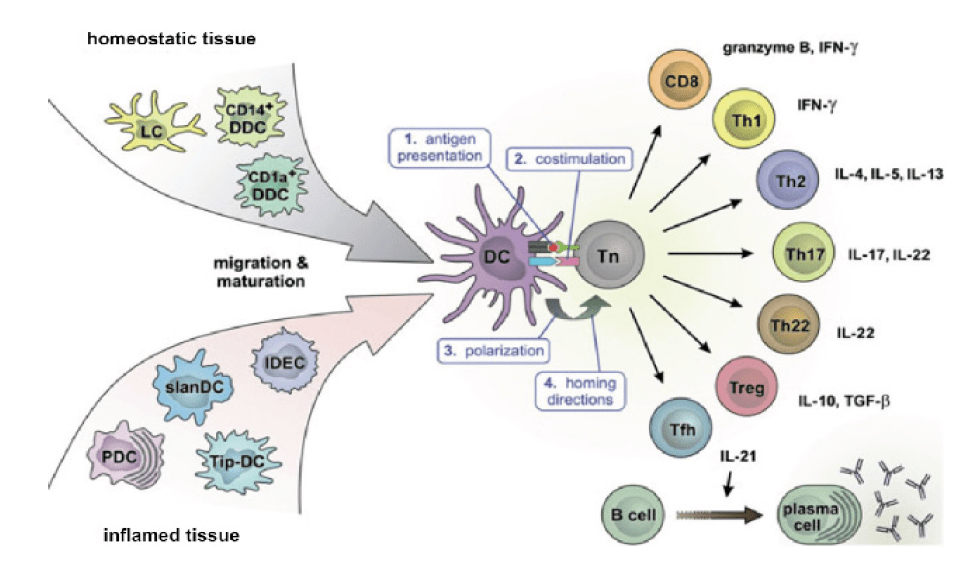
Figure 1:Cellular mechanism of immunomodulation in the cortex of a lymph node draining the area of dendritic cell - antigen encounter.
For immunomodulation from sensitization to tolerance it’s desirable to have vaccines incapable of cross-linking mast cell-bound IgE molecules in densities sufficient to trigger degranulation and mediator release. The maximum number of sequential AAs accommodated by the MHC Class II binding grooves in which antigen-presenting cells present antigens to naive T lymphocytes for immunomodulation is nine. This is shorter than the length of peptide needed to cross-link mast cell IgE receptors and trigger anaphylaxis. Overlapping peptide vaccines were developed to induce immunomodulation from sensitization to tolerance to proteins without the vaccine provoking anaphylaxis. They can be manufactured by replicating the AA sequences of the target proteins by solid phase synthesis.
Overlapping peptide vaccines delivered by traditional means sometimes fails to deliver enough “force” to flip the switch at the center of the figure for successful immunomodulation. An overlapping peptide vaccine for cat allergy (ToleroMune-cat, Circassia since licensed to Adiga Lifesciences) failed to achieve clinical trial objectives. The above analysis of our poison ivy experience suggests that the immunomodulatory “force” of any vaccine could be amplified by delivery to a volume of a recipient tissue with an appropriate combination of dendritic cell types and cytokine milieu in the form of particles sized for dendritic cell uptake by macropinocytosis. This immunomodulatory potency of overlapping peptide vaccines can be increased by giving them solubility properties suitable for VDBP.
Use of overlapping peptides is a shotgun approach to be sure significant linear epitopes are included when the exact amino acid (AA) sequence of relevant linear epitopes is not known. When the exact sequence of relevant linear epitopes is known, only peptides containing those epitopes need be included, reducing the total mass of peptides needed for an effective dose of vaccine. Reducing peptide mass will reduce vaccine viscosity which may improve ability to achieve precipitation in the target particle size range. If Berglund et al were correct in concluding that most (and they suggest all) discontinuous (I. e., conformational) epitopes are composed of short linear epitope sequences forming a binding region for the antibody [13], either overlapping or specific peptide vaccines containing these AA sequences will be capable of immunomodulation if delivered with sufficient potency. Our experience with poison ivy suggests that VDBP is a sufficiently effective potency amplifier to be worth exploring for protein allergens, as well.
Peptides can be manufactured by solid phase synthesis [14,15] in almost any desired combination of AA sequence and chain length. With currently available technology the process can be automated: Program a desired sequence into a device to perform the necessary steps in sequence and at the end you will have the desired peptide. Antigenically significant AA sequences can be incorporated into peptides with desired solubility properties by sandwiching those informational AA sequences between non-informational sequences of either hydrophilic AAs to confer solubility in water or hydrophobic AAs to confer insolubility in water with solubility in one or more of ethanol, acetonitrile and DMSO.
Achievement of the desired solubilities will probably require that the non-informational solubility-modifying AA sequences at each end of each informational AA sequence be longer than the informational AA sequence between them. The total AA chain length of these solubility-modified peptides will thus always be greater than the 5 nM estimated minimum distance between epitopes needed for IgE cross-linking and mediator release [16]. The folded length following VDBP will often be greater than 5 nM, as well. Random human donor serum may contain IgE antibody against randomly generated peptide sequences [17]. There is also a risk that randomly generated peptide sequences might be cross-reactive with normal host proteins and risk induction of autoimmune sensitization. Both of these risks can be minimized by using monomeric AA sequences for non-informational solubility modification to the greatest degree possible. When folding of the non-informational solubility-modifying sequences is needed to achieve targets for either solubility or viscosity we recommend that only a second single hydrophobic AA be used to achieve that configuration and that to the greatest degree possible this second hydrophobic AA should be inserted in monomeric sequences, as well. The goal of these measures is to provide the smallest possible number of potentially tissue-cross-reactive epitopes in the non-informational portion of the proposed solubility-modified peptide vaccines in order to minimize the risk of autosensitization.
The 3D structure of almost any peptide with 5 to 50 AAs can be modeled with resources such as RPBS PEP-FOLD, (on-line at
Informational overlapping peptide size: When target epitope sequences are known, the peptide sets to be used as vaccines need only contain all of the 9 AA sequences (the capacity of the MHC II binding groove [10] included in those epitopes. When all relevant epitope sequences and locations are not known, use of longer overlapping peptides will reduce the mass of extraneous material that must be included for a vaccine to contain all potentially relevant 9 AA sequences. A single peptide 18 AAs long will contain 10 unique 9-AA sequences, for example, while it would require 10 separate peptides each 9 AAs long in addition to their solubility-modifying “tails” in length to provide the same epitope diversity. Eliminating extraneous content will also reduce vaccine viscosity with the above-cited benefit of more rapid dilution by the water content of the recipient tissue, yielding larger numbers of smaller particles. Ethanol and acetonitrile can only draw on the water content of interstitial fluid for dilution. DMSO, which can penetrate phospholipid cell membranes, can also access intracellular water for dilution.
For optimal MHC Class II binding and antigen presentation of epitopes in sequences more than 9 AAs long, tighter MHC class II protein binding resulting in a greater efficacy is achieved if the adjacent AA at each end of the 9 AA sequence presented for immunological recognition is hydrophilic [14]. Vaccine developers cannot control the AAs adjacent to internal 9 AA epitope candidates in overlapping peptides more than 9 AAs in length (in addition to their C-terminal and N-terminal hydrophobic AA tails). However, they can insert a hydrophilic AA at each end of the overlapping peptide sequence of the parent protein, between it and the solubility-modifying strings of hydrophobic AAs at either side.
Candidate Allergens for VDBP Peptide Vaccines for Immunomodulation from Sensitization to Tolerance
The authors’ proposed criteria for candidate allergens for VDBP vaccines for immunomodulation from sensitization to tolerance are that the disease is significant, offending allergens are known, and there is either no currently safe and effective vaccine or current vaccines are not cost-effective. Candidate classes of allergens meeting these criteria include:
• Major food protein allergens for IgE-mediated food allergies.
• Major food protein allergens for non-IgE-mediated food allergies. Including both food-allergic eczema and food-allergic eosinophilic esophagitis (the latter group including food-allergic eczema and food-allergic gastrointestinal diseases).
• Tissue protein antigens of autoimmune diseases
Stinging and biting insect venom allergens and common environmental inhalant aeroallergens are second tier candidates that already have safe and effective treatments. They might be candidates for VDBP after the technology is standardized, if VDBP vaccines for the above-listed first tier candidate allergens prove safer and more effective than current methods of immunotherapy for these second tier candidates, and when the cost of making solubility-modified VDBP vaccines drops to the point at which VDBP vaccines for these second tier allergens would also be cost-competitive.
• Macromolecular protein occupational allergens
Low molecular weight occupational allergens that act as haptens are not proteins and therefore not candidates for the peptide vaccine VDBP that’s the major topic of this review. However, their interaction with the host immune system is identical to that of the urushiols of PO and PI except that for volatile haptens of which an example is toluene diisocyanate the primary target tissue is usually the lung. For non-volatile urushols the target tissue is almost always the skin.
Diisocyanates are small molecular occupational allergens encountered in the polymerization of polyurethanes in both manufacturing and the application of polyurethane paints and surface sealers. They bind to tissue proteins and act as haptens [18] much like PI and PO, and are also insoluble in water and soluble in most organic solvents. This makes them potential candidates for VDBP using the same methods developed for PI [4] with no need for the methods described above for epitopes of proteins.
Candidate Allergens for Immunomodulation from Tolerance (In Cancer and Some Infectious Diseases) or Naivete (In Other Infectious Diseases) to Protective Sensitization
• This is also the authors’ personal “ToDo list.”
• Every cancer patient’s individual tumor-specific antigens.
• Epidemic/pandemic viral infections including COVID-19, other epidemic coronaviruses and other viruses including Ebola, Zika, influenza, insect-born viral encephalitides, others.
• Malaria and other epidemiologically significant parasitic diseases for which current treatments are sub-optimal.
• Tuberculosis and other treatment-resistant mycobacterial infections.
• Tick-borne rickettsial diseases.
Choice of Epitopes to Exclude From ILIT-Adapted Overlapping Peptide Vaccines
When the complete AA sequence of clinically relevant epitopes is known, overlapping peptides that do not contain AA sequences of known relevant epitopes can be excluded from the vaccine. When multiple relevant epitopes are known, molecular modeling may guide which combinations are likely to be effective with the minimun total mass of solubility-modified peptide, maximizing the ratio of relevant epitope content to viscosity. Reducing irrelevant peptide content will reduce viscosity and facilitate ability to achieve rates of solvent dilution yielding particles in the target size range of 0.5 to 5 microns. When the objective is immunomodulation from naivete or tolerance to sensitization (in cancer or infectious diseases), exclusion of epitopes replicating the structure of receptors or agonists of human metabolic processes will reduce or eliminate the risk of anti-idiotypic antibody-mediated adverse effects [19]. Inclusion of either enough known epitopes to achieve efficacy or of large numbers of overlapping peptides as a shotgun approach to the same goal may increase viscosity enough that administration in a single injection will miss the particle size range of 0.5 to 5 microns. This can be accommodated by the same expedient we employed with our more dilute poison ivy vaccines, of dividing the dose between multiple individual injections closely enough spaced to have a high probability of serving the same draining lymph nodes.
Choice of solvent, target tissue and method of administration:
The combination of ethanol as a solvent and skeletal muscle as a target tissue was safe and effective for immunomodulation from sensitization to tolerance in allergy to poison ivy, with 0.15 ml maximum volumes of one or more physically closely spaced individual injections. Multiple injections were sometimes required to achieve target treatment dose with less concentrated vaccines to fit our arbitrarily chosen maximum ethanol volume of 0.15 ml per injection. When multiple injections were needed they were given in close proximity in the same target tissue to maximize the likelihood that particles of antigen scavenged by dendritic cells trafficking the locations in which they were precipitated would be delivered and presented to naive T cells in the same lymph node or same set of lymph nodes. Multiple injections may be needed for overlapping peptide vaccines to keep vaccine viscosity low enough to achieve a rate of solvent dilution that precipitates particles in the 0.5 to 5 micron size range for dendritic cell uptake by macropinocytosis.
Substitution of pharmaceutically acceptable volumes of low viscosity acetonitrile for ethanol as a vaccine vehicle for injection into skeletal muscle may reduce vaccine viscosity, increase rate of solvent dilution, and reduce precipitated particle size in applications in which ethanol yields particles that are too large. For vaccines for which the most effective solvent is acetonitrile, cumulative injected volume is a potential source of toxicity because the metabolism of acetonitrile releases cyanide. Even if need to limit viscosity forces dilution into as large a cumulative injected volume of 2 ml (13 separate injections of 0.15 ml), the cumulative injected dose of acetonitrile will be well below any threshold for toxicity suggested by the currently posted EPA Toxicological Review [20].
The viscosity of DMSO as a single component solvent may be too high to achieve effective particle size distribution for VDBP on injection into muscle. Combinations of DMSO with either ethanol or acetonitrile may allow effective VDBP for vaccines that are not adequately soluble in ethanol or acetonitrile alone. Topically applied vaccines in DMSO may be capable of effective vaccine particle size delivery by a mechanism independent of its viscosity, because of its ability to penetrate and carry dissolved solute across biological phospholipid membranes including intact skin. A wave of topically applied DMSO will diffuse across both cellular and tissue phospholipid membranes carrying with it dissolved solute. Movement of the solute front will be slowed by what is essentially tissue chromatography as the solvent is also diluted by tissue water. Vaccine carried across phospholipid membrane by the diffusion of topically applied DMSO will become insoluble and precipitate at a more rapid rate than if the same vaccine was delivered by injection of a viscous DMSO bolus because the mass of DMSO that diffuses across those membranes and must then be diluted to achieve precipitation is so much less.
The size and location of precipitated particles of VDBP vaccines formulated for IM or intradermal injection can be traced by injection of small volumes into a the corresponding tissue of a mouse, euthanization and electron microscopy of the area surrounding the injection site. Particle size and location following topical application to the skin in DMSO, topical application to the nasal mucosa in any of the three solvents or intradermal administration with various intradermal injection devices can be similarly tracked by application to those tissues of a mouse, again followed by euthanizing and electron microscopy (E/M). The glutaraldehyde fixative used for E/M will cross-link peptides in place [21,22], producing a demarcated homogeneous mass that should be easy to identify and measure. If particle size is above target, viscosity can be reduced by reducing total peptide concentration of which viscosity is an exponential function [23] and by altering the composition of the solubility-modifying hydrophobic AA end-chains to reduce intermolecular interactions. Solubility-modifying chains of physically smaller hydrophobic AAs glycine and alanine may have less of a stearic contribution to viscosity than chains larger and bulkier AAs
Tissues in which the primary evolutionary role of the immune system is protection against invading pathogens are more likely to be populated by sensitizing lineages of dendritic cells and have lymph node cytokine milieus that favor protective sensitization. These include the dermis and the lining of the nasal mucosa. Vaccines in DMSO applied topically to either skin or nasal mucosa may achieve effective vaccine precipitation rates to produce particles appropriately sized for macropinocytosis. Vaccines dissolved in any of the three solvents may be delivered to the dermis by injection or with any of a number of existing intradermal vaccine injection technologies [24].
Throughout our discussion of VDBP we have avoided calling the process of populating a target tissue with precipitated particles “atraumatic,” as exposure to any of the three solvents will cause some degree of transient chemical shock. That shock may be sufficiently disruptive of the integrity of the nasal mucosal barrier to enable effective topical delivery of vaccines dissolved in ethanol or acetonitrile by spraying from a metered dose pump. Vaccines dissolved in more viscous DMSO can be similarly applied by brushing or as drops. The nasal mucosa is a particularly attractive site for immunization against infectious diseases for which the nasal mucosa is the primary portal of entry, such as COVID-19, for which the induction of local cell-mediated immunity can reduce both infection and transmission. For COVID-19 there are good animal models in which some [25-27] but not all [28] intranasally applied vaccines have induced protective immunity.
Potential Adjuvant Effect of a Concurrent, Co-Localized Cell-Mediated (Poison Ivy-Like) Hypersensitivity Reaction
The presence of inflammation induces different populations of dendritic cells, as illustrated in the lower left hand corner of the figure. The deliberate induction of a cell-mediated hypersensitivity reaction in the same tissue either prior to or concurrent with overlapping peptide VDBP will increase influx of dendritic cells of lineages associated with allergic sensitization and may further bias the response toward protective sensitization. Sensitizers appropriate for this use should be universal, so that all or nearly all recipients will respond. They should be non-natural, so that no recipients would be naturally sensitized and at unrecognized risk for more severe reactions because of prior sensitization to doses determined to be safe and effective in previously unsensitized recipients. Use of the historical standard sensitizer of this class, dinitrochlorobenzene (DNCB) is controversial because it fails the Ames test for mutagenicity [29]. However, its clinical efficacy as an inducer of protective sensitization against the tumor-inducing virus of verruca vulgaris raises questions about whether its mutagenicity on the Ames in fact indicates a risk of carcinogenicity in human use. Alternative Ames test-negative universal sensitizers have been proposed [29].
The adjuvant effect of an induced cell-mediated hypersensitivity reaction on induction of protective sensitization for infectious diseases can be measured in animal models that mimic both human susceptibility to the pathogen and human sensitivity to the external sensitizer. Because almost every cancer patient’s tumor antigens are unique, the adjuvant effect of induced cell mediated hypersensitivity reactions on cancer immunotherapy can best be studied in randomized clinical trials. Variables that may impact the added efficacy of an induced cell mediated reaction include timing with respect to vaccine delivery and intensity of the induced cell mediated reaction. Persons previously sensitized to a non-natural sensitizer as an adjuvant for vaccine efficacy may need a lower dose than unsensitized individuals for an adjuvant reaction of the same intensity if ever needed for either a different vaccine or for a booster. For this reason we recommend World Health Organization involvement in the choice of a single internationally accepted non-natural sensitizer as a VDBP sensitizing vaccine adjuvant. Previous exposure to this sensitizer can then be documented in each patient’s vaccine history record, with the caveat that some cancer patients may be anergic and need higher doses.
Additional Considerations tor VDBP Vaccines for SARS Co-V2
VDBP SARS Co-V2 vaccines could be made with solubility-modified peptides whose informational segments contain the AA sequences of potentially or confirmed immunogenic T-cell epitopes of any proteins accessible to the host immune system. They could be on either or both of the surfaces of viral particles or the surfaces of infected cells. When exact epitope AA sequences are known, only solubility-modified peptides containing those informational sequences need be included. When the exact epitope AA sequences are not known, overlapping peptide informational segments can cover them. In any case we recommend not including epitopes that bind with or emulate elements of the ACE-2 receptor to minimize risk of autoimmune adverse effects.
In 2021, Pan et al reported identification of 4 epitopes on the SARS Co-V2 non-structural protein helicase (NSP13) and one on the membrane glycoprotein (MGP) that could be eluted from naturally processed major histocompatability complex and then demonstrated to induce the production of cytotoxic T-cells specific for the same epitopes [30]. The authors report that these 5 epitopes are coded by a highly conserved region of the SARS Co-V2 genome unchanged between the original pandemic strain and all variants available for study through mid-June 2021. This constancy suggests that solubility-modified peptide vaccines indcorporating these and other similarly discovered epitopes that are equally conserved will retain effectiveness against new variants emerging under the same environmental selective pressures.
Solubility-modified versions of the peptide sequences of these 5 epitopes, synthesized as informational sequences between non-informational hydrophobic strings of hydrophobic AAs to confer the combination of insolubility in water with solubility in one or more of ethanol, acetonitrile and DMSO, collectively comprise a high probability formulation for a safe and effective VDBP Sars Co-V2 vaccine.
Conclusions
We learned from poison ivy that precipitation of a water-insoluble antigen into 0.5 to 5 micron particles within the body of a non-liquid recipient tissue, vaccine delivery by precipitation or VDBP, is a potent and efficient way to feed that antigen to APCs and enhance its ability to induce therapeutic immunomodulation.
We outline methods to adapt VDBP to clinically relevant epitopes of protein antigens. We do this for both therapeutic immunomodulation from sensitization to tolerance in allergic diseases and from tolerance in cancer and from naivete or tolerance in infectious diseases to protective sensitization.
We review potential obstacles to the achievement of these goals. We review methods to track the efficacy of vaccine delivery to its intended location and to measure its particle size. We then discuss the specific example of a VDBP peptide vaccine for SARS CoV2.
Conflict of Interest
We the authors discovered the phenomenon of VDBP, interpreted its probable mechanism of action, and have patents either issued or applications pending for uses discussed in this article.
We thank the editors for this opportunity to present our findings and our ideas for other ways to exploit the immunomodulatory potency of this method of feeding antigen to the immune system.
References
1. Epstein WL. Occupational poison ivy and oak dermatitis. Dermatol Clin. 1994;12(3):511-6.
2. Watson S. Toxicodendron hyposensitization programs. Clin Dermatol.1986;4(2):160-170.
3. Kim Y, Flamm A, ElSohly M, et al. Poison Ivy, Oak, and Sumac Dermatitis: What Is Known and What Is New? Dermatitis. 2019;30(3):183-190. doi: 10.1097/DER.0000000000000472.
4. Coifman RE, Yang CF. Tolerance to poison ivy following vaccine delivery by precipitation, Annals of Allergy, Asthma Immunol. 2019;122:331-33.
5. Xiang SD, Scholzen A, Minigo G, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1e9.
6. Teunissen MBM, Haniffa M, Collin MP. Insight into the Immunobiology of Human Skin and Functional Specialization of Skin Dendritic Cell Subsets to Innovate Intradermal Vaccination Design. In Teunissen MBM, editor, Intradermal Immunization. Current Topics Microbiol Immunol. 351: 113-138.
7. O’mahony L, Akdis M, Crameri R, et al. Novel immunotherapeutic approaches for allergy and asthma. Autoimmunity, November 2010; 43(7): 493–503 q Informa UK, Ltd. ISSN 0891-6934 print/1607-842X online DOI: 10.3109/08916931003674725
8. Neimert-Andersson T, Thunberg S, Swedin L, et al. Carbohydrate-based particles reduce allergic inflammation in a mouse model for cat allergy. Allergy. 2008; 63:518-526.
9. Kovacsovics-Bankowski M, Clark K, Benacerraf B, et al. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Nail Acad Sci USA. 1993;90:4942-4946.
10. Arnold PY, La Gruta NL, Miller T, et al. The majority of immunogenic epitopes generate CD4+ T cells that are dependent on MHC class II-bound peptide-flanking residues. J Immunol. 2002;169(2):739-49. doi: 10.4049/jimmunol.169.2.739
11. Kane PM, Holowka D, Baird B. Cross-linking of IgE-Receptor Complexes by Rigid Bivalent Antigens >200 A in Length Triggers Cellular Degranulation. J Cell Biol. 1988;107: 969-980.
12. Göbl C, et al. Flexible IgE epitope containing domains of Phl p 5 cause high allergenic activity. J Allergy Clin Immunol. 2017; 140(4): 1187–1191. doi:10.1016/j.jaci.2017.05.005.
13. Berglund L, Andrade J, Odeberg J, et al. The epitope space of the human proteome. Protein Sci. 2008, 17:606–613.
14. Stawikowski M, Fields GB. Introduction to Peptide Synthesis. Curr Protoc Protein Sci. 2002; CHAPTER: Unit–18.1. doi:10.1002/0471140864.ps1801s26.
15. Coin I, Beyermann M, Bienert M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat Protoc. 2007; 2: 3247–3256 (2007). https://doi.org/10.1038/nprot.2007.454
16. Knol EF. Requirements for effective IgE cross-linking on mast cells and basophils. Mol Nutr Food Res. 2006; 50: 620 – 624 DOI 10.1002/mnfr.200500272.
17. Krause T, et al. IgE Epitope Profiling for Allergy Diagnosis and Therapy – Parallel Analysis of a Multitude of Potential Linear Epitopes Using a High Throughput Screening Platform. Front Immunol. 2020. | https://doi.org/10.3389/fimmu.2020.565243
18. Nakashima K, Takeshita T & Morimoto K: Review of the Occupational Exposure to Isocyanates, Mechanisms of Action. Environmental Health and Preventive Medicine 2002;7:1–6.
19. Murphy WJ, Longo DL. A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. New England J Medicine. doi.org/10.1056/NEJMcibr2113694.
20. EPA: Toxicological revoew opf aceonoitrile (CAS No. 75-05-8), January 1999, https://iris.epa.gov/static/pdfs/0205tr.pdf
21. Mascorro JA, Bozzola JJ. Processing biological tissues for ultrastructural study. Methods Mol Biol. 2007;369:19-34.
22. Migneault I, Dartiguenave C, Bertrand MJ, et al. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques. 2004; 37:790-802.
23. Gonçalves AD, Alexander C, Roberts CJ, et al. The effect of protein concentration on the viscosity of a recombinant albumin solution formulation. RSC Advances. 2016; 6” 15143-15154.
24. Kim YC, Jarrahian C, Zehrung D, et al. Delivery Systems for Intradermal Vaccination In Teunissen MBM, editor, Intradermal Immunization. Current Topics in Microbiol Immunol. 351: 76-112.
25. van Doremalen N, et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. bioRxiv preprint doi: https://doi.org/10.1101/2021.01.09.426058.
26. Hassan AO, et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell. 2020;183169–184.
27. Hassan AO et al. A single intranasal dose of chimpanzee adenovirus vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Reports Med. 2021;2:100230.
28. Furuyama W et al. Rapid Protection from COVID-19 in Nonhuman Primates Vaccinated Intramuscularly but Not Intranasally with a Single Dose of a Vesicular Stomatitis Virus-Based Vaccine. 2022; 13 (issue 1) e03379-21.
29. Happle R. The Potential Hazards of Dinitrochlorobenzene. Arch Dermatol. (1985)121:330-1.
30. Pan K, et al. Mass spectrometric identification of immunogenic SARS-CoV-2 epitopes and cognate TCRs. Proc Nat Acad Sci. 2021; 118 (46) e2111815118.
Received: March 01, 2022;
Accepted: March 28, 2022;
Published: March 29, 2022.
To cite this article : Coifman RE, Yang CF. Vaccine Delivery by Precipitation (VDBP) Lessons from Poison Ivy for Protein Antigens in General and Specifically for SARS Co-V2 European Journal of Respiratory Medicine. 2022; 4(2): 290 - 305. doi: 10.31488/EJRM.130.
© 2022 Coifman RE, et al.
